Abstract
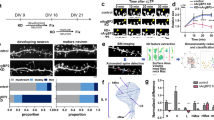
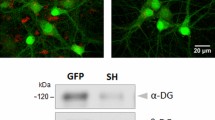
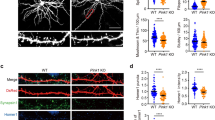
Mutations of the cyclin-dependent kinase-like 5 (CDKL5) and netrin-G1 (NTNG1) genes cause a severe neurodevelopmental disorder with clinical features that are closely related to Rett syndrome, including intellectual disability, early-onset intractable epilepsy and autism. We report here that CDKL5 is localized at excitatory synapses and contributes to correct dendritic spine structure and synapse activity. To exert this role, CDKL5 binds and phosphorylates the cell adhesion molecule NGL-1. This phosphorylation event ensures a stable association between NGL-1 and PSD95. Accordingly, phospho-mutant NGL-1 is unable to induce synaptic contacts whereas its phospho-mimetic form binds PSD95 more efficiently and partially rescues the CDKL5-specific spine defects. Interestingly, similarly to rodent neurons, iPSC-derived neurons from patients with CDKL5 mutations exhibit aberrant dendritic spines, thus suggesting a common function of CDKL5 in mice and humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bienvenu, T. & Chelly, J. Molecular genetics of Rett syndrome: when DNA methylation goes unrecognized. Nat. Rev. Genet. 7, 415–426 (2006).
Chahrour, M. & Zoghbi, H. Y. The story of Rett syndrome: from clinic to neurobiology. Neuron 56, 422–437 (2007).
Amir, R. E. et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 23, 185–188 (1999).
Francke, U. Mechanisms of disease: neurogenetics of MeCP2 deficiency. Nat. Clin. Pract. Neurol. 2, 212–221 (2006).
Tao, J. et al. Mutations in the X-linked cyclin-dependent kinase-like 5 (CDKL5/STK9) gene are associated with severe neurodevelopmental retardation. Am. J. Hum. Genet. 75, 1149–1154 (2004).
Weaving, L. S. et al. Mutations of CDKL5 cause a severe neurodevelopmental disorder with infantile spasms and mental retardation. Am. J. Hum. Genet. 75, 1079–1093 (2004).
Archer, H. L. et al. CDKL5 mutations cause infantile spasms, early onset seizures, and severe mental retardation in female patients. J. Med. Genet. 43, 729–734 (2006).
Kalscheuer, V. M. et al. Disruption of the serine/threonine kinase 9 gene causes severe X-linked infantile spasms and mental retardation. Am. J. Hum. Genet. 72, 1401–1411 (2003).
Córdova-Fletes, C. et al. CDKL5 truncation due to a t(X;2)(p22.1;p25.3) in a girl with X-linked infantile spasm syndrome. Clin. Genet. 77, 92–96 (2010).
Scala, E. et al. CDKL5/STK9 is mutated in Rett syndrome variant with infantile spasms. J. Med. Genet. 42, 103–107 (2005).
Rademacher, N. et al. Identification of a novel CDKL5 exon and pathogenic mutations in patients with severe mental retardation, early-onset seizures and Rett-like features. Neurogenetics 12, 165–167 (2011).
Bahi-Buisson, N. et al. Key clinical features to identify girls with CDKL5 mutations. Brain 131, 2647–2661 (2008).
Van Esch, H., Jansen, A., Bauters, M., Froyen, G. & Fryns, J. P. Encephalopathy and bilateral cataract in a boy with an interstitial deletion of Xp22 comprising the CDKL5 and NHS genes. Am. J. Med. Genet. A 143, 364–369 (2007).
Mei, D. et al. Xp22.3 genomic deletions involving the CDKL5 gene in girls with early onset epileptic encephalopathy. Epilepsia 51, 647–654 (2010).
Liang, J. S. et al. CDKL5 alterations lead to early epileptic encephalopathy in both genders. Epilepsia 52, 1835–1842 (2011).
Bertani, I. et al. Functional consequences of mutations in CDKL5, an X-linked gene involved in infantile spasms and mental retardation. J. Biol. Chem. 281, 32048–32056 (2008).
Montini, E. et al. Identification and characterization of a novel serine-threonine kinase gene from the Xp22 region. Genomics 51, 427–433 (1988).
Mari, F. et al. CDKL5 belongs to the same molecular pathway of MeCP2 and it is responsible for the early-onset seizure variant of Rett syndrome. Hum. Mol. Genet. 14, 1935–1946 (2005).
Rusconi, L. et al. CDKL5 expression is modulated during neuronal development and its subcellular distribution is tightly regulated by the C-terminal tail. J. Biol. Chem 283, 30101–30111 (2008).
Ricciardi, S., Kilstrup-Nielsen, C., Bienvenu, T., Jacquette, A., Landsberger, N. & Broccoli, V. CDKL5 influences RNA splicing activity by its association to the nuclear speckle molecular machinery. Hum. Mol. Genet. 18, 4590–4602 (2009).
Chen, Q. et al. CDKL5, a protein associated with Rett syndrome, regulates neuronal morphogenesis via Rac1 signaling. J. Neurosci. 30, 12777–12786 (2010).
Rusconi, L., Kilstrup-Nielsen, C. & Landsberger, N. Extrasynaptic N-methyl-D-aspartate (NMDA) receptor stimulation induces cytoplasmic translocation of the CDKL5 kinase and its proteasomal degradation. J. Biol. Chem. 286, 36550–36558 (2011).
Borg, I. et al. Disruption of Netrin G1 by a balanced chromosome translocation in a girl with Rett syndrome. Euro. J. Human Genet. 13, 921–927 (2005).
Woo, J., Kwon, S. K. & Kim, E. The NGL family of leucine-rich repeat-containing synaptic adhesion molecules. Mol. Cell Neurosci. 42, 1–10 (2009).
Kim, E. & Sheng, M. The postsynaptic density. Curr. Biol. 19, R723–R724 (2009).
Lin, J. C., Ho, W. H., Gurney, A. & Rosenthal, A. The netrin-G1 ligand NGL-1 promotes the outgrowth of thalamocortical axons. Nat. Neurosci. 6, 1270–1276 (2003).
Kim, S. et al. NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat. Neurosci. 9, 1294–1301 (2006).
Huttlin, E. L. et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 (2010).
Migaud, M. et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396, 433–439 (1998).
Rosas-Vargas, H. et al. Impairment of CDKL5 nuclear localisation as a cause for severe infantile encephalopathy. J. Med. Genet. 45, 172–178 (2008).
Kim, K. Y., Hysolli, E. & Park, I. H. Neuronal maturation defect in induced pluripotent stem cells from patients with Rett syndrome. Proc. Natl Acad. Sci. USA 108, 14169–14174 (2011).
Marchetto, M. C. et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143, 527–539 (2010).
Tchieu, J. et al. Female human iPSCs retain an inactive X chromosome. Cell Stem. Cell 7, 329–342 (2010).
Colleoni, S. et al. Long-term culture and differentiation of CNS precursors derived from anterior human neural rosettes following exposure to ventralizing factors. Exp. Cell Res. 316, 1148–1158 (2010).
Bayés, A. et al. Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nat. Neurosci. 14, 19–21 (2011).
Hering, H. & Sheng, M. Dendritic spines: structure, dynamics and regulation. Nat. Rev. Neurosci. 2, 880–888 (2001).
Woo, J. et al. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat. Neurosci. 12, 428–437 (2009).
Han, K. & Kim, E. Synaptic adhesion molecules and PSD-95. Prog. Neurobiol. 84, 263–283 (2008).
Van Kuilenburg, A. B. et al. Analysis of severely affected patients with dihydropyrimidine dehydrogenase deficiency reveals large intragenic rearrangements of DPYD and a de novo interstitial deletion del(1)(p13.3p21.3). Hum. Genet. 125, 581–90 (2009).
O’Roak, B. J. et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485, 246–250 (2012).
Ohtsuki, T. et al. Association of polymorphisms in the haplotype block spanning the alternatively spliced exons of the NTNG1 gene at 1p13.3 with schizophrenia in Japanese populations. Neurosci. Lett. 435, 194–197 (2008).
Cohen, N. A., Brenman, J. E., Snyder, S. H. & Bredt, D. S. Binding of the inward rectifier K+ channel Kir 2.3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron 17, 759–767 (1996).
Chung, H. J., Huang, Y. H., Lau, L. F. & Huganir, R. L. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J. Neurosci. 24, 10248–10259 (2004).
Essmann, C. L. et al. Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat. Neurosci. 11, 1035–1043 (2008).
Kennedy, M. B., Beale, H. C., Carlisle, H. J. & Washburn, L. R. Integration of of biochemical signalling in spines. Nat. Rev. Neurosci. 6, 423–434 (2005).
Acknowledgements
We gratefully acknowledge T. Bienvenu for providing primary fibroblasts from CDKL5 patients, H. Van Esch and H. Archer for providing DNA samples from patients, S. Freier, A. Walther, A. Grimme, U. Fischer and B. Moser for their excellent technical assistance, and L. Musante for helpful discussions. L. Pecciarini is acknowledged for karyotype analysis of the iPSC lines. This study was supported by the Telethon Foundation, Ministry of Health, IIT-SEED project, EuroRETT network to V.B. and the EU-FP7 project GENCODYS (241995) to V.M.K.
Author information
Authors and Affiliations
Contributions
V.M.K. and N.R. initiated this study. S.R., M.H., N.R, V.M.K. and V.B. contributed to its conceptualization and V.M.K and V.B. directed it throughout; S.R. performed and analysed neuronal experiments. F.U. generated iPSCs and characterized iPSC-derived neurons. M.H. performed and analysed immunoprecipitation assays and Phos-tag assays. M.H. and N.R. performed mutation search. G.S. was involved in mutagenesis experiments. D.B. performed electrophysiological recordings. A.S. performed electroporation experiments. C.M. and A.B. performed 2D gel analysis. E.G. produced the CDKL5-specific antibody. C.V. and C.S. assisted with the preparation of primary neurons. C.K-N. assisted with the in vitro phosphorylation assays. V.M.K., V.B., S.R., M.H. and N.R. interpreted the results. V.B., V.M.K. and S.R. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4704 kb)
Rights and permissions
About this article
Cite this article
Ricciardi, S., Ungaro, F., Hambrock, M. et al. CDKL5 ensures excitatory synapse stability by reinforcing NGL-1–PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons. Nat Cell Biol 14, 911–923 (2012). https://doi.org/10.1038/ncb2566
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2566
This article is cited by
-
mGluR5 PAMs rescue cortical and behavioural defects in a mouse model of CDKL5 deficiency disorder
Neuropsychopharmacology (2023)
-
Research progress on the pathogenesis of CDKL5 pathogenic variants and related encephalopathy
European Journal of Pediatrics (2023)
-
Signalling pathways in autism spectrum disorder: mechanisms and therapeutic implications
Signal Transduction and Targeted Therapy (2022)
-
Expression of a Secretable, Cell-Penetrating CDKL5 Protein Enhances the Efficacy of Gene Therapy for CDKL5 Deficiency Disorder
Neurotherapeutics (2022)
-
Novel Cross-Correction–Enabled Gene Therapy for CDKL5-Deficiency Disorder
Neurotherapeutics (2022)