Abstract
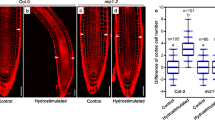
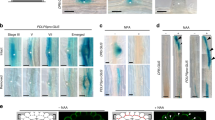
Aquaporins are membrane channels that facilitate water movement across cell membranes. In plants, aquaporins contribute to water relations. Here, we establish a new link between aquaporin-dependent tissue hydraulics and auxin-regulated root development in Arabidopsis thaliana. We report that most aquaporin genes are repressed during lateral root formation and by exogenous auxin treatment. Auxin reduces root hydraulic conductivity both at the cell and whole-organ levels. The highly expressed aquaporin PIP2;1 is progressively excluded from the site of the auxin response maximum in lateral root primordia (LRP) whilst being maintained at their base and underlying vascular tissues. Modelling predicts that the positive and negative perturbations of PIP2;1 expression alter water flow into LRP, thereby slowing lateral root emergence (LRE). Consistent with this mechanism, pip2;1 mutants and PIP2;1-overexpressing lines exhibit delayed LRE. We conclude that auxin promotes LRE by regulating the spatial and temporal distribution of aquaporin-dependent root tissue water transport.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Casimiro, I. et al. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 8, 165–71 (2003).
Péret, B., Larrieu, A. & Bennett, M. J. Lateral root emergence: a difficult birth. J. Exp. Bot. 60, 3637–43 (2009).
Péret, B. et al. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 14, 399–408 (2009).
Overvoorde, P., Fukaki, H. & Beeckman, T. Auxin control of root development. Cold Spring Harb. Perspect. Biol. 2, a001537 (2010).
Swarup, K. et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10, 946–54 (2008).
Cosgrove, D. J. Water uptake by growing cells: an assessment of the controlling roles of wall relaxation, solute uptake, and hydraulic conductance. Int. J. Plant Sci. 154, 10–21 (1993).
Boyer, J. S. & Silk, W. K. Hydraulics of plant growth. Funct. Plant Biol. 31, 761–773 (2004).
Ehlert, C., Maurel, C., Tardieu, F. & Simonneau, T. Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiol. 150, 1093–104 (2009).
Fricke, W. Biophysical limitation of cell elongation in cereal leaves. Ann. Bot. 90, 157–67 (2002).
Hukin, D., Doering-Saad, C., Thomas, C. R. & Pritchard, J. Sensitivity of cell hydraulic conductivity to mercury is coincident with symplasmic isolation and expression of plasmalemma aquaporin genes in growing maize roots. Planta 215, 1047–56 (2002).
Oparka, K. J., Prior, D. A. M. & Wright, K. M. Symplastic communication between primary and developing lateral roots of Arabidopsis thaliana. J. Exp. Bot. 46, 187–197 (1995).
Gomes, D. et al. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim. Biophys. Acta 1788, 1213–28 (2009).
Danielson, J. A. & Johanson, U. Phylogeny of major intrinsic proteins. Adv. Exp. Med. Biol. 679, 19–31 (2010).
Kaldenhoff, R. et al. Aquaporins and plant water balance. Plant Cell Environ. 31, 658–66 (2008).
Maurel, C., Verdoucq, L., Luu, D. T. & Santoni, V. Plant aquaporins: membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 59, 595–624 (2008).
Ditengou, F. A. et al. Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 105, 18818–23 (2008).
Laskowski, M. et al. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 6, 2721–2735 (2008).
Lucas, M., Godin, C., Jay-Allemand, C. & Laplaze, L. Auxin fluxes in the root apex co-regulate gravitropism and lateral root initiation. J. Exp. Bot. 59, 55–66 (2008).
Richter, G. L., Monshausen, G. B., Krol, A. & Gilroy, S. Mechanical stimuli modulate lateral root organogenesis. Plant Physiol. 151, 1855–66 (2009).
Malamy, J. E. & Benfey, P. N. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124, 33–44 (1997).
Benková, E. et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602 (2003).
Alexandersson, E. et al. Whole gene family expression and drought stress regulation of aquaporins. Plant Mol. Biol. 59, 469–84 (2005).
Monneuse, J. M. et al. Towards the profiling of the Arabidopsis thaliana plasma membrane transportome by targeted proteomics. Proteomics 11, 1789–97 (2011).
Calderon-Villalobos, L. I., Tan, X., Zheng, N. & Estelle, M. Auxin perception–structural insights. Cold Spring Harb. Perspect. Biol. 2, a005546 (2010).
Okushima, Y. et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17, 444–63 (2005).
Okushima, Y., Fukaki, H., Onoda, M., Theologis, A. & Tasaka, M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19, 118–30 (2007).
Wilmoth, J. C. et al. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 43, 118–30 (2005).
De Smet, I. et al. Bimodular auxin response controls organogenesis in Arabidopsis. Proc. Natl Acad. Sci. USA 107, 2705–10 (2010).
Santoni, V., Vinh, J., Pflieger, D., Sommerer, N. & Maurel, C. A proteomic study reveals novel insights into the diversity of aquaporin forms expressed in the plasma membrane of plant roots. Biochem J. 373, 289–96 (2003).
Javot, H. et al. Role of a single aquaporin isoform in root water uptake. Plant Cell 15, 509–22 (2003).
Boursiac, Y. et al. Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization. Plant J. 56, 207–18 (2008).
Kazama, H. & Katsumi, M. The role of the osmotic potential of the cell in auxin-induced cell elongation. Plant Cell Physiol. 19, 1145 (1978).
Da Ines, O. et al. Kinetic analyses of plant water relocation using deuterium as tracer-reduced water flux of Arabidopsis pip2 aquaporin knockout mutants. Plant Biol. (Stuttg) 12 (Suppl. 1), 129–39 (2010).
Laskowski, M., Biller, S., Stanley, K., Kajstura, T. & Prusty, R. Expression profiling of auxin-treated Arabidopsis roots: toward a molecular analysis of lateral root emergence. Plant Cell Physiol. 47, 788–92 (2006).
Wisman, E., Cardon, G. H., Fransz, P. & Saedler, H. The behaviour of the autonomous maize transposable element En/Spm in Arabidopsis thaliana allows efficient mutagenesis. Plant Mol. Biol. 37, 989–99 (1998).
Tissier, A. F. et al. Multiple independent defective suppressor-mutator transposon insertions in Arabidopsis: a tool for functional genomics. Plant Cell 11, 1841–52 (1999).
Alonso, J. M. et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657 (2003).
Scholl, R. L., May, S. T. & Ware, D. H. Seed and molecular resources for Arabidopsis. Plant Physiol. 124, 1477–1480 (2000).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–43 (1998).
Geldner, N. et al. Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J. 59, 169–78 (2009).
Postaire, O. et al. A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis. Plant Physiol. 152, 1418–30 (2010).
Boursiac, Y. et al. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 139, 790–805 (2005).
Péret, B. et al. Auxin influx activity is associated with Frankia infection during actinorhizal nodule formation in Casuarina glauca. Plant Physiol. 144, 1852–62 (2007).
Acknowledgements
This work was supported by a Marie Curie Intra-European Fellowship within the 7th European Community Framework Programme PIEF-GA-2008-220506 (B.P.) and by a Grand Federative Project (Rhizopolis) of the Agropolis Fondation (Montpellier, France) to C.M. We are indebted to H. Scherb (Helmholtz Zentrum München) for his help with statistical analyses. L.R.B., U.V., M.L., D.M.W., J.R.K., O.E.J. and M.J.B. acknowledge the support of the Biotechnology and Biological Sciences Research Council (BBSRC) and Engineering and Physical Sciences Research Council (EPSRC) funding to the Centre for Plant Integrative Biology (CPIB), BBSRC responsive mode grant support to U.V., L.R.B. and M.J.B. and the BBSRC Professorial Research Fellowship funding to D.M.W. and M.J.B. A.R.S. and O.D.I. acknowledge the support of the Deutsche Forschungsgemeinschaft priority programme SPP1108 (SCHA 454/8).
Author information
Authors and Affiliations
Contributions
B.P., G.L., J.Z., U.V., O.P., D-T.L., O.D.I., I.C., M.L., D.M.W., L.L. and P.N. performed experimental work; B.P., G.L., J.Z., L.R.B., J.R.K., O.E.J., A.R.S., C.M. and M.J.B. performed data analysis; B.P., L.R.B., J.R.K., O.E.J., A.R.S., C.M. and M.J.B. oversaw project planning; B.P., L.R.B., A.R.S., C.M. and M.J.B. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1165 kb)
Supplementary Table 1
Supplementary Information (XLS 50 kb)
Supplementary Note
Supplementary Information (PDF 511 kb)
Supplementary Data
Supplementary Information (ZIP 3 kb)
Rights and permissions
About this article
Cite this article
Péret, B., Li, G., Zhao, J. et al. Auxin regulates aquaporin function to facilitate lateral root emergence. Nat Cell Biol 14, 991–998 (2012). https://doi.org/10.1038/ncb2573
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2573
This article is cited by
-
Water relations in plants treated with growth promoting rhizosphere bacteria
Plant and Soil (2024)
-
Auxin- and pH-induced guttation in Phycomyces sporangiophores: relation between guttation and diminished elongation growth
Protoplasma (2023)
-
Melatonin Application at Different Doses Changes the Physiological Responses in Favor of Cabbage Seedlings (Brassica oleracea var. capitata) Against Flooding Stress
Gesunde Pflanzen (2023)
-
Plasma membrane aquaporins of the PIP1 and PIP2 subfamilies facilitate hydrogen peroxide diffusion into plant roots
BMC Plant Biology (2022)
-
Molecular Cloning and Functional Characterization of FOUR LIPS in Pomegranate, a Protein Involved in Regulating the Gravitropic Set-Point Angle of Adventitious Roots
Journal of Plant Biology (2022)