Abstract
The rising incidence of obesity and related disorders such as diabetes and heart disease has focused considerable attention on the discovery of new therapeutics. One promising approach has been to increase the number or activity of brown-like adipocytes in white adipose depots, as this has been shown to prevent diet-induced obesity and reduce the incidence and severity of type 2 diabetes. Thus, the conversion of fat-storing cells into metabolically active thermogenic cells has become an appealing therapeutic strategy to combat obesity. Here, we report a screening platform for the identification of small molecules capable of promoting a white-to-brown metabolic conversion in human adipocytes. We identified two inhibitors of Janus kinase (JAK) activity with no precedent in adipose tissue biology that stably confer brown-like metabolic activity to white adipocytes. Importantly, these metabolically converted adipocytes exhibit elevated UCP1 expression and increased mitochondrial activity. We further found that repression of interferon signalling and activation of hedgehog signalling in JAK-inactivated adipocytes contributes to the metabolic conversion observed in these cells. Our findings highlight a previously unknown role for the JAK–STAT pathway in the control of adipocyte function and establish a platform to identify compounds for the treatment of obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bartelt, A. et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205 (2011).
Stanford, K. I. et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123, 215–223 (2013).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Ghorbani, M. & Himms-Hagen, J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int. J. Obes. Relat. Metab. Disord. 21, 465–475 (1997).
Granneman, J. G., Li, P., Zhu, Z. & Lu, Y. Metabolic and cellular plasticity in white adipose tissue I: effects of β3-adrenergic receptor activation. Am. J. Physiol. Endocrinol. Metab. 289, E608–E616 (2005).
Himms-Hagen, J. et al. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am. J. Physiol. Cell Physiol. 279, C670–C681 (2000).
Li, P., Zhu, Z., Lu, Y. & Granneman, J. G. Metabolic and cellular plasticity in white adipose tissue II: role of peroxisome proliferator-activated receptor-α. Am. J. Physiol. Endocrinol. Metab. 289, E617–E626 (2005).
Koh, Y. J. et al. Activation of PPAR γ induces profound multilocularization of adipocytes in adult mouse white adipose tissues. Exp. Mol. Med. 41, 880–895 (2009).
Murano, I., Barbatelli, G., Giordano, A. & Cinti, S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J. Anat. 214, 171–178 (2009).
Petrovic, N. et al. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285, 7153–7164 (2010).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Rosenwald, M., Perdikari, A., Rulicke, T. & Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15, 659–667 (2013).
Van der Lans, A. A. et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 123, 3395–3403 (2013).
Yoneshiro, T. et al. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 123, 3404–3408 (2013).
Ahfeldt, T. et al. Programming human pluripotent stem cells into white and brown adipocytes. Nat. Cell Biol. 14, 209–219 (2012).
Bonet, M. L., Oliver, P. & Palou, A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim. Biophys. Acta 1831, 969–985 (2013).
Kozak, U. C. et al. An upstream enhancer regulating brown-fat-specific expression of the mitochondrial uncoupling protein gene. Mol. Cell. Biol. 14, 59–67 (1994).
Rubio, A., Raasmaja, A., Maia, A. L., Kim, K. R. & Silva, J. E. Effects of thyroid hormone on norepinephrine signaling in brown adipose tissue. I. β 1- and β 2-adrenergic receptors and cyclic adenosine 3’,5’-monophosphate generation. Endocrinology 136, 3267–3276 (1995).
Rubio, A., Raasmaja, A. & Silva, J. E. Thyroid hormone and norepinephrine signaling in brown adipose tissue. II: differential effects of thyroid hormone on β 3-adrenergic receptors in brown and white adipose tissue. Endocrinology 136, 3277–3284 (1995).
Rabelo, R., Schifman, A., Rubio, A., Sheng, X. & Silva, J. E. Delineation of thyroid hormone-responsive sequences within a critical enhancer in the rat uncoupling protein gene. Endocrinology 136, 1003–1013 (1995).
Mocsai, A., Ruland, J. & Tybulewicz, V. L. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat. Rev. Immunol. 10, 387–402 (2010).
Richard, A. J. & Stephens, J. M. The role of JAK-STAT signaling in adipose tissue function. Biochim. Biophys. Acta 1842, 431–439 (2014).
Meyer, D. M. et al. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J. Inflamm. 7, 41–51 (2010).
Flanagan, M. E. et al. Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J. Med. Chem. 53, 8468–8484 (2010).
Braselmann, S. et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J. Pharmacol. Exp. Ther. 319, 998–1008 (2006).
Cao, W., Medvedev, A. V., Daniel, K. W. & Collins, S. β-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. J. Biol. Chem. 276, 27077–27082 (2001).
Cao, W. et al. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 24, 3057–3067 (2004).
Valladares, A., Roncero, C., Benito, M. & Porras, A. TNF-α inhibits UCP-1 expression in brown adipocytes via ERKs. Opposite effect of p38MAPK. FEBS Lett. 493, 6–11 (2001).
Aaronson, D. S. & Horvath, C. M. A road map for those who don’t know JAK-STAT. Science 296, 1653–1655 (2002).
Todoric, J. et al. Cross-talk between interferon-γ and hedgehog signaling regulates adipogenesis. Diabetes 60, 1668–1676 (2011).
Pospisilik, J. A. et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell 140, 148–160 (2010).
Derecka, M. et al. Tyk2 and Stat3 regulate brown adipose tissue differentiation and obesity. Cell Metab. 16, 814–824 (2012).
Bray, M. A. & Carpenter, A. et al. in Assay Guidance Manual (ed Sittampalam, G. S.) (Bethesda, 2004).
Prummer, M. Hypothesis testing in high-throughput screening for drug discovery. J. Biomol. Screen. 17, 519–529 (2012).
Storey, J. D. A direct approach to false discovery rates. J. Royal Stat. Soc. 64, 479–498 (2002).
Acknowledgements
The authors thank I. Clausen, M. Kapps, R. Schmucki and A. Schuler for technical support, K. Christensen and M. Graf for stem cell support, L. Badi for preliminary data analysis, C. Solier, A. Schell-Steven and T. Bergauer for experimental planning and M. Pawlak (Natural and Medical Sciences Institute at the University of Tübingen) for RPPA analyses. A.M. was supported by the Roche Postdoctoral Fellowship (RPF) program (2011–2013). This research was supported in part by F. Hoffmann-La Roche; grant R01DK095384 (C.A.C. and Y.K.L.) and R01DK097768 (C.A.C.) from the United States Institutes of Health (NIH); and Harvard University.
Author information
Authors and Affiliations
Contributions
A.M. designed and performed experiments, analysed data and wrote the manuscript; Y-K.L. performed experiments, analysed data and edited the manuscript; R.G., C.S.H. and F.X. performed experiments; J.D.Z. and M.E. performed bioinformatics analyses and contributed to the main text related to Fig. 7; H.H.T., S.Z. and M.P. performed high-content imaging analysis; A.K. performed RNA-seq; C.A.M. and R.T.S. supervised stem cell activities; K.E.A. supervised the project and C.A.C. supervised the project and wrote the manuscript. A.M., Y-K.L., R.G., C.S.H., M.P., J.D.Z., H.H.T., S.Z. and A.K. contributed to description of Methods.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 3
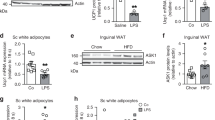
(a) UCP1 expression is increased in PSC-WA after treatment with indicated compounds. Rosiglitazone induced expression of both UCP1 and FABP4. Values represent the mean of two biological replicates. (b) Browning screen performance: Plate-wise control distribution and Z’ factors for UCP1 and FABP4. For UCP1, <Z’ >= 0.50, for FABP4, <Z’ >= 0.64. (c) Reproducibility of replicates. The correlation of the intra-plate replicates for UCP1 (left) and FABP4 (right) in log–log representation. Controls are plotted in red (negative) and green (positive). Compounds with large inter-run differences were excluded from further analysis. (d) Hit selection. Left panel: Quantile–quantile plot of the normalized UCP1 reads. The blue line indicates the expected profile for a Gaussian distribution without actives. The blue dashed lines limit the confidence band of a correlation test corroborating the hit selection from a different angle, as all the selected compounds (green dots) lie outside. The P-value distribution drawn in the inset, which is the basis of the hit selection, shows no irregular features. Right panel: Scatter plot of the normalized FABP4 and UCP1 signal on logarithmic axes. Black dots: inactive compounds. Color dots: active compounds, red: rosiglitazone, yellow: rosiglitazone-like compounds in screen, blue: potential browning hits in screen, green: rosiglitazone-like compounds and browning hits confirmed in validation run, grey : non-confirmed in validation run.
Supplementary Figure 4 PSC-WA were differentiated and treated as described in Fig. 1b.
At day 14, adipocytes were fixed, stained and imaged by confocal microscopy. Green: lipids, Red: nuclei. Scale bars: 50 mM.
Supplementary Figure 6
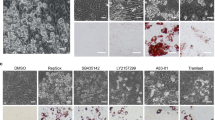
(a) Uncropped bright field images showing that tofacitinib and R406 induce brown-like lipid morphology in human primary adipocytes more prominently than BMP7. ADSC: Adipose tissue-derived stem cells. (b) RT-PCR analysis of UCP1 expression in mouse visceral white adipose tissue explants following 7 days of treatment with tofacitinib (tofa.) or R406. Values are mean ± s.e.m. of n = three biological replicates of pooled tissue from 5 mice.
Supplementary Figure 7 Reverse phase protein analysis (RPPA) of tofacitinib and R406-treated PSC-WA.
Cells were collected 20’ after addition of compounds to PSC-WA and processed for RPPA. Data shown is relative to DMSO. All 51 antibodies recognized the phospho-isoforms of indicated proteins. 3 antibodies did not reach detection threshold and were excluded from graph. Data from one experiment (n = 3 biological replicates) representative of 2 independent experiments. Values are mean ± s.d. of three biological replicates.
Supplementary Figure 8
(a) R406 promoted mRNA expression of PGC1α, PGC1β and PPARG but not of PRDM16. (b) The transcriptional changes downstream of R406 include up-regulation of PPARG, SREBF and BMP target genes. (c) Gene expression regulation by tofacitinib (tofa., JAK3i) versus R406 (SYKi) in PSC-WA at day 7. 54 genes were up-regulated by both compounds, 17 of which were BA-specific, that is, low in PSC-WA and high in PSC-BA (yellow dots). (a–c) N = 3 biological replicates. Each independent biological replicate was pooled from two individual wells.
Supplementary Figure 9 The ratio of UCP1/FABP4 mRNA level was down-regulated in PSC-BA upon treatment with cyclopamine.
Values represent the mean of two biological replicates.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2148 kb)
Source Data
Supplementary Information (XLSX 427 kb)
Rights and permissions
About this article
Cite this article
Moisan, A., Lee, YK., Zhang, J. et al. White-to-brown metabolic conversion of human adipocytes by JAK inhibition. Nat Cell Biol 17, 57–67 (2015). https://doi.org/10.1038/ncb3075
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3075
This article is cited by
-
Deep learning enables the quantification of browning capacity of human adipose samples
Journal of Big Data (2024)
-
The effects of suppressing inflammation by tofacitinib may simultaneously improve glycaemic parameters and inflammatory markers in rheumatoid arthritis patients with comorbid type 2 diabetes: a proof-of-concept, open, prospective, clinical study
Arthritis Research & Therapy (2024)
-
Altered macronutrient composition and genetics influence the complex transcriptional network associated with adiposity in the Collaborative Cross
Genes & Nutrition (2022)
-
Signaling pathways in obesity: mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2022)
-
Januskinaseinhibitoren
Zeitschrift für Rheumatologie (2022)