Abstract
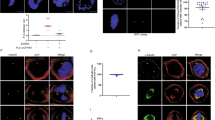
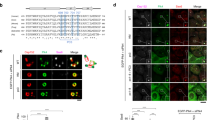
We show that human Cdc14A phosphatase1 interacts with interphase centrosomes, and that this interaction is independent of microtubules and Cdc14A phosphatase activity, but requires active nuclear export. Disrupting the nuclear export signal (NES) led to Cdc14A being localized in nucleoli, which in unperturbed cells selectively contain Cdc14B (ref. 1). Conditional overproduction of Cdc14A, but not its phosphatase-dead or NES-deficient mutants, or Cdc14B, resulted in premature centrosome splitting and formation of supernumerary mitotic spindles. In contrast, downregulation of endogenous Cdc14A by short inhibitory RNA duplexes (siRNA) induced mitotic defects including impaired centrosome separation and failure to undergo productive cytokinesis. Consequently, both overexpression and downregulation of Cdc14A caused aberrant chromosome partitioning into daughter cells. These results indicate that Cdc14A is a physiological regulator of the centrosome duplication cycle, which, when disrupted, can lead to genomic instability in mammalian cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Li, L., Ernsting, B. R., Wishart, M. J., Lohse, D. L. & Dixon, J. E. J. Biol. Chem. 272, 29403–29406 (1997).
Shou, W. et al. Cell 97, 233–244 (1999).
Visintin, R., Hwang, E. S. & Amon, A. Nature 398, 818–823 (1999).
Trautmann, S. et al. Curr. Biol. 11, 931–940 (2001).
Cueille, N. et al. J. Cell Sci. 114, 2649–2664 (2001).
Lukas, J., Sørensen, C. S., Lukas, C., Santoni-Rugiu, E. & Bartek, J. Oncogene 18, 3930–3935 (1999).
Hinchcliffe, E. H. & Sluder, G. Genes Dev. 15, 1167–1181 (2001).
Mayor, T., Meraldi, P., Stierhof, Y. D., Nigg, E. A. & Fry, A. M. FEBS Lett. 452, 92–95 (1999).
Tassin, A. M. & Bornens, M. Biol Cell 91, 343–354 (1999).
Mayor, T., Stierhof, Y. D., Tanaka, K., Fry, A. M. & Nigg, E. A. J. Cell Biol. 151, 837–846 (2000).
White, J. & Stelzer, E. Trends Cell Biol. 9, 61–65 (1999).
Fukuda, M. et al. Nature 390, 308–311 (1997).
Henderson, B. R. & Eleftheriou, A. Exp. Cell Res. 256, 213–224 (2000).
Hagting, A., Karlsson, C., Clute, P., Jackman, M. & Pines, J. EMBO J. 17, 4127–4138 (1998).
Visintin, R. & Amon, A. Curr. Opin. Cell Biol. 12, 372–377 (2000).
Piel, M., Nordberg, J., Euteneuer, U. & Bornens, M. Science 291, 1550–1553 (2001).
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Nature 396, 643–649 (1998).
Clute, P. & Pines, J. Nature Cell Biol. 1, 82–87 (1999).
Elbashir, S. M. et al. Nature 411, 494–498 (2001).
Acknowledgements
We thank M. Bornens, H. Charbonneau, G. Evan, E. Nigg and M. Yoshida for reagents, and the Danish Cancer Society, Human Frontier Science Programme, the John and Birthe Meyer Foundation, and the National Institutes of Health (grants GM54811, GM60439) for financial support.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Movie 1
Transient overexpression of Cdc14A induces generation of multiple daughter cells. U-2-OS-Cdc14A(wt) cells were synchronized by doublethymidime treatment, released, and induced to express the transgene. After 14 h, the cells were subjected to time-lapse videomicroscopy. Elapsed time (hours:minutes) is indicated in the upper right corner. Scale bar is indicated in the first frame. (MOV 1818 kb)
Movie 2
Impaired cytokinesis after the siRNA-mediated dowregulation of Cdc14A. HeLa cells were transfected by the siRNA duplexes (100 nM) directed to the bases 89-109 of the human Cdc14A coding sequence and subjected to time-lapse videomicroscopy. Recording was initiated 48 h after transfection and the elapsed time (hours:minutes) is indicated in the upper right corner. This dividing cell underwent anaphase, attempted to generate a cleavage furrow but failed to execute a productive cytokinesis. As a result, the cell re-entered a G1-like state with doubled DNA content. Scale bar is indicated in the first frame. (MOV 1545 kb)
Movie 3
Delayed cell fusion after the siRNA-mediated downregulation of Cdc14A. HeLa cells were transfected by the siRNA duplexes (100 nM) directed to the bases 89-109 of the human Cdc14A coding sequence and subjected to time-lapse videomicroscopy. Recording was initiated 48 h after transfection and the elapsed time (hours:minutes) is indicated in the upper right corner. This cell underwent a rapid and apparently normal anaphase and telophase, but the two emerging daughter cells remained connected by a narrow cytoplasmic bridge with a discernible, central located midbody. The lack of its productive abscission led eventually to a cytoplasmic fusion and generation of a bi-nuclear cell. Scale bar is indicated in the first frame. (MOV 2884 kb)
Figure 1
Transient overexpression of Cdc14A induces formation of multipolar mitotic spindle. U-2-OS-Cdc14A(wt) cells were synchronized by doublethymidine treatment, released, and induced to express the transgene. After 14 h, the cells were fixed and co-immunostained by antibodies to a mitotic spindle motor protein (Eg5) and centriolar marker (centrin). (PDF 104 kb)
Rights and permissions
About this article
Cite this article
Mailand, N., Lukas, C., Kaiser, B. et al. Deregulated human Cdc14A phosphatase disrupts centrosome separation and chromosome segregation. Nat Cell Biol 4, 318–322 (2002). https://doi.org/10.1038/ncb777
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb777
This article is cited by
-
Wip1 controls the translocation of the chromosomal passenger complex to the central spindle for faithful mitotic exit
Cellular and Molecular Life Sciences (2021)
-
Biochemical analyses reveal amino acid residues critical for cell cycle-dependent phosphorylation of human Cdc14A phosphatase by cyclin-dependent kinase 1
Scientific Reports (2018)
-
The anaphase promoting complex impacts repair choice by protecting ubiquitin signalling at DNA damage sites
Nature Communications (2017)
-
Cdc14 phosphatase: warning, no delay allowed for chromosome segregation!
Current Genetics (2016)
-
Mutant p53 drives multinucleation and invasion through a process that is suppressed by ANKRD11
Oncogene (2012)