Abstract
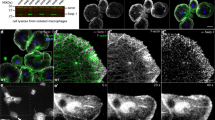
The capacity to repair a wound is a fundamental survival mechanism that is activated at any site of damage throughout embryonic and adult life1. To study the cell biology and genetics of this process, we have developed a wounding model in Drosophila melanogaster embryos that allows live imaging of rearrangements and changes in cell shape, and of the cytoskeletal machinery that draws closed an in vivo wound. Using embryos expressing green fluorescent protein (GFP) fusion proteins, we show that two cytoskeletal-dependent elements — an actin cable and dynamic filopodial/lamellipodial protrusions — are expressed by epithelial cells at the wound edge and are pivotal for repair. Modulating the activities of the small GTPases Rho and Cdc42 demonstrates that these actin-dependent elements have differing cellular functions, but that either alone can drive wound closure. The actin cable operates as a 'purse-string' to draw the hole closed, whereas filopodia are essential for the final 'knitting' together of epithelial cells at the end of repair. Our data suggest a more complex model for epithelial repair than previously envisaged and highlight remarkable similarities with the well-characterized morphogenetic movement of dorsal closure in Drosophila.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Martin, P. Science 276, 75–81 (1997).
Jacinto, A., Martinez-Arias, A. & Martin, P. Nature Cell Biol. 3, E117–E123 (2001).
Martinez-Arias, A. in The Development of Drosophila melanogaster (eds Bate, M. and Martinez-Arias, A.) 517–607 (Cold Spring Harbor Laboratory Press, New York, 1993).
Jacinto, A., Woolner, S. & Martin, P. Dev. Cell 3, 9–19 (2002).
Harden, N. Differentiation 70, 181–203 (2002).
Jacinto, A. et al. Curr. Biol 10, 1420–1426 (2000).
Magie, C. R., Meyer, M. R., Gorsuch, M. S. & Parkhurst, S. M. Development 126, 5353–5364 (1999).
Harden, N., Ricos, M., Ong, Y. M., Chia, W. & Lim, L. J. Cell Sci. 112, 273–284 (1999).
Bloor, J. W. & Kiehart, D. P. Development 129, 3173–3183 (2002).
Jacinto, A. et al. Curr. Biol. 12, 1245–1250 (2002).
Martin, P. & Lewis, J. Nature 360, 179–183 (1992).
Bement, W. M., Forscher, P. & Mooseker, M. S. J. Cell Biol. 121, 565–578 (1993).
Danjo, Y. & Gipson, I. K. J. Cell Sci. 111, 3323–3332 (1998).
Brock, J., Midwinter, K., Lewis, J. & Martin, P. J. Cell Biol. 135, 1097–1107 (1996).
Foe, V. E. Development 107, 1–22 (1989).
Kiehart, D. P., Galbraith, C. G., Edwards, K. A., Rickoll, W. L. & Montague, R. A. J. Cell Biol. 149, 471–490 (2000).
Young, P. E., Richman, A. M., Ketchum, A. S. & Kiehart, D. P. Genes Dev. 7, 29–41 (1993).
Strutt, D. I., Weber, U. & Mlodzik, M. Nature 387, 292–295 (1997).
Ridley, A. J. & Hall, A. Cell 70, 389–399 (1992).
Nobes, C. D. & Hall, A. Cell 81, 53–62 (1995).
Genova, J. L., Jong, S., Camp, J. T. & Fehon, R. G. Dev. Biol. 221, 181–194 (2000).
Hakeda-Suzuki, S. et al. Nature 416, 438–442 (2002).
Ramet, M., Lanot, R., Zachary, D. & Manfruelli, P. Dev. Biol. 241, 145–156 (2002).
Brand, A. H. & Perrimon, N. Development 118, 401–415 (1993).
Verkhusha, V. V., Tsukita, S. & Oda, H. FEBS Lett. 445, 395–401 (1999).
Oda, H. & Tsukita, S. Dev. Genes Evol. 209, 218–225 (1999).
Luo, L., Liao, Y. J., Jan, L. Y. & Jan, Y. N. Genes Dev. 8, 1787–1802 (1994).
Mizuno, T., Tsutsui, K. & Nishida, Y. Development 129, 1215–1223 (2002).
Karnovsky, M. J. J. Cell Biol. 27, 137–138 (1965).
Acknowledgements
We thank all in the Martin lab for constant encouragement and support, K. Barrett and K. Nikolaidou for helpful discussions, M. Turmaine for help with electron microscopy and A. Martinez-Arias for continued enthusiasm and advice. The GFP fusion fly stocks were generously supplied by H. Oda (GFP–actin and α-catenin) and R. Karess (GFP–sqh). The anti-Rho1 antiserum was kindly provided by C. Magie and S. Parkhurst. Thanks also to the Bloomington Stock Center, D. Strutt, B. Dickson and N. Harden for other fly stocks. This work is funded by the Medical Research Council, The Wellcome Trust, The Royal Society and a Pfizer UK studentship to W.W.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Movie 1
Movie 1 shows the final hour of epithelial repair in a wild type embryo expressing GFP-actin. (MOV 2762 kb)
Movie 2
Movie 2 illustrates repair of a mechanically-created incisional wound made to an embryo expressing GFP-actin. (MOV 2163 kb)
Movie 3
Movie 3 reveals actin dynamics during wound closure in a Rho mutant embryo expressing GFP-actin. (MOV 2270 kb)
Movie 4
Movie 4 shows a direct comparison of wild type (right) and Rho mutant (left) wound closure in embryos expressing GFP-actin. (MOV 4667 kb)
Movie 5
Movie 5 is a direct comparison of wild type (right) and Cdc42N17-expressing (left) wounds. (MOV 3257 kb)
Legends
Legends to movies (PDF 12 kb)
Rights and permissions
About this article
Cite this article
Wood, W., Jacinto, A., Grose, R. et al. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol 4, 907–912 (2002). https://doi.org/10.1038/ncb875
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb875
This article is cited by
-
Combined computational modeling and experimental study of the biomechanical mechanisms of platelet-driven contraction of fibrin clots
Communications Biology (2023)
-
Epithelial wound healing in Clytia hemisphaerica provides insights into extracellular ATP signaling mechanisms and P2XR evolution
Scientific Reports (2023)
-
Rab11 negatively regulates wingless preventing JNK-mediated apoptosis in Drosophila epithelium during embryonic dorsal closure
Cell and Tissue Research (2023)
-
The first embryo, the origin of cancer and animal phylogeny. II. The neoplastic process as an evolutionary engine
Journal of Biosciences (2023)
-
The analytical solution to the migration of an epithelial monolayer with a circular spreading front and its implications in the gap closure process
Biomechanics and Modeling in Mechanobiology (2023)