Abstract
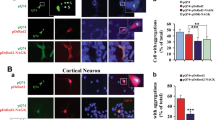
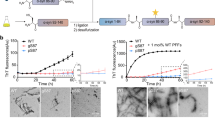
Several aggregation-prone proteins associated with neurodegenerative diseases can be modified by O-linked N-acetyl-glucosamine (O-GlcNAc) in vivo. One of these proteins, α-synuclein, is a toxic aggregating protein associated with synucleinopathies, including Parkinson's disease. However, the effect of O-GlcNAcylation on α-synuclein is not clear. Here, we use synthetic protein chemistry to generate both unmodified α-synuclein and α-synuclein bearing a site-specific O-GlcNAc modification at the physiologically relevant threonine residue 72. We show that this single modification has a notable and substoichiometric inhibitory effect on α-synuclein aggregation, while not affecting the membrane binding or bending properties of α-synuclein. O-GlcNAcylation is also shown to affect the phosphorylation of α-synuclein in vitro and block the toxicity of α-synuclein that was exogenously added to cells in culture. These results suggest that increasing O-GlcNAcylation may slow the progression of synucleinopathies and further support a general function for O-GlcNAc in preventing protein aggregation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hardivillé, S. & Hart, G. W. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab. 20, 208–213 (2014).
Bond, M. R. & Hanover, J. A. A little sugar goes a long way: the cell biology of O-GlcNAc. J. Cell Biol. 208, 869–880 (2015).
Yuzwa, S. A. & Vocadlo, D. J. O-GlcNAc and neurodegeneration: biochemical mechanisms and potential roles in Alzheimer's disease and beyond. Chem. Soc. Rev. 43, 6839–6858 (2014).
Zhu, Y., Shan, X., Yuzwa, S. A. & Vocadlo, D. J. The emerging link between O-GlcNAc and Alzheimer disease. J. Biol. Chem. 289, 34472–34481 (2014).
O'Donnell, N., Zachara, N. E., Hart, G. W. & Marth, J. D. OGT-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol. Cell. Biol. 24, 1680–1690 (2004).
Lefebvre, T. et al. Evidence of a balance between phosphorylation and O-GlcNAc glycosylation of Tau proteins—a role in nuclear localization. Biochim. Biophys. Acta 1619, 167–176 (2003).
Liu, F., Iqbal, K., Grundke-Iqbal, I., Hart, G. & Gong, C. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer's disease. Proc. Natl Acad. Sci. USA 101, 10804–10809 (2004).
Wang, Z. et al. Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol. Cell. Proteom. 9, 153–160 (2010).
Alfaro, J. F. et al. Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets. Proc. Natl Acad. Sci. USA 109, 7280–7285 (2012).
Yuzwa, S. A. et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nature Chem. Biol. 4, 483–490 (2008).
Yuzwa, S. A. et al. Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nature Chem. Biol. 8, 393–399 (2012).
Martí, M. J., Tolosa, E. & Campdelacreu, J. Clinical overview of the synucleinopathies. Mov. Disord. 18 (Suppl. 6), S21–S27 (2003).
Lashuel, H. A., Overk, C. R., Oueslati, A. & Masliah, E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nature Rev. Neurosci. 14, 38–48 (2013).
Emanuele, M. & Chieregatti, E. Mechanisms of alpha-synuclein action on neurotransmission: cell-autonomous and non-cell autonomous role. Biomolecules 5, 865–892 (2015).
Fink, A. L. The aggregation and fibrillation of α-synuclein. Acc. Chem. Res. 39, 628–634 (2006).
George, S., Rey, N. L., Reichenbach, N., Steiner, J. A. & Brundin, P. α-Synuclein: the long distance runner. Brain Pathol. 23, 350–357 (2013).
Recasens, A. & Dehay, B. Alpha-synuclein spreading in Parkinson's disease. Front. Neuroanat. 8, 159 (2014).
Brettschneider, J., Tredici, K. D., Lee, V. M.-Y. & Trojanowski, J. Q. Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nature Rev. Neurosci. 16, 109–120 (2015).
Wang, Z. et al. Site-specific GlcNAcylation of human erythrocyte proteins potential biomarker(s) for diabetes. Diabetes 58, 309–317 (2009).
Marotta, N. P., Cherwien, C. A., Abeywardana, T. & Pratt, M. R. O-GlcNAc modification prevents peptide-dependent acceleration of α-synuclein aggregation. ChemBioChem 13, 2665–2670 (2012).
Yuzwa, S. A. et al. Mapping O-GlcNAc modification sites on tau and generation of a site-specific O-GlcNAc tau antibody. Amino Acids 40, 857–868 (2011).
Cheung, W. D., Sakabe, K., Housley, M. P., Dias, W. B. & Hart, G. W. O-linked beta-N-acetylglucosaminyltransferase substrate specificity is regulated by myosin phosphatase targeting and other interacting proteins. J. Biol. Chem. 283, 33935–33941 (2008).
Oueslati, A., Fournier, M. & Lashuel, H. A. Role of post-translational modifications in modulating the structure, function and toxicity of alpha-synuclein: implications for Parkinson's disease pathogenesis and therapies. Prog. Brain Res. 183, 115–145 (2010).
Koo, H.-J., Choi, M. Y. & Im, H. Aggregation-defective alpha-synuclein mutants inhibit the fibrillation of Parkinson's disease-linked alpha-synuclein variants. Biochem. Biophys. Res. Commun. 386, 165–169 (2009).
Coelho-Cerqueira, E., Pinheiro, A. S. & Follmer, C. Pitfalls associated with the use of Thioflavin-T to monitor anti-fibrillogenic activity. Bioorg. Med. Chem. Lett. 24, 3194–3198 (2014).
Blanco-Canosa, J. B. & Dawson, P. E. An efficient Fmoc-SPPS approach for the generation of thioester peptide precursors for use in native chemical ligation. Angew. Chem. Int. Ed. 47, 6851–6855 (2008).
Lorenzen, N. et al. The role of stable α-synuclein oligomers in the molecular events underlying amyloid formation. J. Am. Chem. Soc. 136, 3859–3868 (2014).
Paslawski, W. et al. High stability and cooperative unfolding of α-synuclein oligomers. Biochemistry 53, 6252–6263 (2014).
Jao, C. C., Hegde, B. G., Chen, J., Haworth, I. S. & Langen, R. Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement. Proc. Natl Acad. Sci. USA 105, 19666–19671 (2008).
Varkey, J. et al. Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins. J. Biol. Chem. 285, 32486–32493 (2010).
Mizuno, N. et al. Remodeling of lipid vesicles into cylindrical micelles by α-synuclein in an extended α-helical conformation. J. Biol. Chem. 287, 29301–29311 (2012).
Boassa, D. et al. Mapping the subcellular distribution of α-synuclein in neurons using genetically encoded probes for correlated light and electron microscopy: implications for Parkinson's disease pathogenesis. J. Neurosci. 33, 2605–2615 (2013).
Vargas, K. J. et al. Synucleins regulate the kinetics of synaptic vesicle endocytosis. J. Neurosci. 34, 9364–9376 (2014).
Paleologou, K. E. et al. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J. Biol. Chem. 283, 16895–16905 (2008).
Paleologou, K. E. et al. Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J. Neurosci. 30, 3184–3198 (2010).
Mbefo, M. K. et al. Phosphorylation of synucleins by members of the Polo-like kinase family. J. Biol. Chem. 285, 2807–2822 (2010).
Oueslati, A., Schneider, B. L., Aebischer, P. & Lashuel, H. A. Polo-like kinase 2 regulates selective autophagic α-synuclein clearance and suppresses its toxicity in vivo. Proc. Natl Acad. Sci. USA 110, E3945–E3954 (2013).
Tabrizi, S. J. et al. Expression of mutant alpha-synuclein causes increased susceptibility to dopamine toxicity. Hum. Mol. Genet. 9, 2683–2689 (2000).
Lee, M., Hyun, D., Halliwell, B. & Jenner, P. Effect of the overexpression of wild-type or mutant alpha-synuclein on cell susceptibility to insult. J. Neurochem. 76, 998–1009 (2001).
Ko, L.-W., Ko, H.-H. C., Lin, W.-L., Kulathingal, J. G. & Yen, S.-H. C. Aggregates assembled from overexpression of wild-type alpha-synuclein are not toxic to human neuronal cells. J. Neuropathol. Exp. Neurol. 67, 1084–1096 (2008).
Khalaf, O. et al. The H50Q mutation enhances α-synuclein aggregation, secretion, and toxicity. J. Biol. Chem. 289, 21856–21876 (2014).
Olanow, C. W. & Brundin, P. Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder? Mov. Disord. 28, 31–40 (2013).
Chen, M., Margittai, M., Chen, J. & Langen, R. Investigation of alpha-synuclein fibril structure by site-directed spin labeling. J. Biol. Chem. 282, 24970–24979 (2007).
Vilar, M. et al. The fold of alpha-synuclein fibrils. Proc. Natl Acad. Sci. USA 105, 8637–8642 (2008).
Gambetta, M. C. & Müller, J. O-GlcNAcylation prevents aggregation of the Polycomb group repressor polyhomeotic. Dev. Cell 31, 629–639 (2014).
Hejjaoui, M., Haj-Yahya, M., Kumar, K. S. A., Brik, A. & Lashuel, H. A. Towards elucidation of the role of ubiquitination in the pathogenesis of Parkinson's disease with semisynthetic ubiquitinated α-synuclein. Angew. Chem. Int. Ed. 50, 405–409 (2011).
Haj-Yahya, M. et al. Synthetic polyubiquitinated α-synuclein reveals important insights into the roles of the ubiquitin chain in regulating its pathophysiology. Proc. Natl Acad. Sci. USA 110, 17726–17731 (2013).
Hejjaoui, M. et al. Elucidating the role of C-terminal post-translational modifications using protein semisynthesis strategies: α-synuclein phosphorylation at tyrosine 125. J. Am. Chem. Soc. 134, 5196–5210 (2012).
Fauvet, B. et al. Characterization of semisynthetic and naturally Nα-acetylated α-synuclein in vitro and in intact cells: implications for aggregation and cellular properties of α-synuclein. J. Biol. Chem. 287, 28243–28262 (2012).
Burai, R., Ait-Bouziad, N., Chiki, A. & Lashuel, H. A. Elucidating the role of site-specific nitration of α-synuclein in the pathogenesis of Parkinson's disease via protein semisynthesis and mutagenesis. J. Am. Chem. Soc. 137, 5041–5052 (2015).
Acknowledgements
B.W.Z. is a fellow of the National Science Foundation Graduate Research Fellowship Program (DGE-0937362). This research was supported by the Michael J. Fox Foundation (M.R.P.), the National Institutes of Health (R01GM063915 to R.L. and R01NS081678 to D.B.A.) and in part by the US National Cancer Institute of the US National Institutes of Health (CCSG P30CA014089). Some circular dichroism and dynamic light scattering measurement were performed at the USC Nano Biophysics Core Facility. TEM of protein fibres was performed at the USC Center for Electron Microscopy and Microanalysis.
Author information
Authors and Affiliations
Contributions
N.P.M., Y.H.L., Y.E.L., M.R.A., B.W.Z., M.R., D.B.A., R.L. and M.R.P. designed experiments and interpreted data. N.P.M. carried out the synthesis of the synuclein proteins, performed aggregation reactions and collected circular dichroism and dynamic light scattering spectroscopy data, ThT measurements and TEM images. Y.H.L. performed cell-death assays. Y.E.L. performed mutant synuclein experiments. M.R.A. performed membrane-binding experiments. B.W.Z. performed SDS–PAGE analysis. M.R. collected primary neurons. N.P.M., Y.H.L., Y.E.L. and M.R.P. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2799 kb)
Rights and permissions
About this article
Cite this article
Marotta, N., Lin, Y., Lewis, Y. et al. O-GlcNAc modification blocks the aggregation and toxicity of the protein α-synuclein associated with Parkinson's disease. Nature Chem 7, 913–920 (2015). https://doi.org/10.1038/nchem.2361
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2361
This article is cited by
-
Unravelling cell type-specific responses to Parkinson’s Disease at single cell resolution
Molecular Neurodegeneration (2024)
-
Glycosylation and behavioral symptoms in neurological disorders
Translational Psychiatry (2023)
-
O-GlcNAcylation determines the translational regulation and phase separation of YTHDF proteins
Nature Cell Biology (2023)
-
O-GlcNAcylation regulates neurofilament-light assembly and function and is perturbed by Charcot-Marie-Tooth disease mutations
Nature Communications (2023)
-
Post-translational modifications of soluble α-synuclein regulate the amplification of pathological α-synuclein
Nature Neuroscience (2023)