Abstract
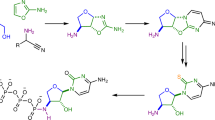
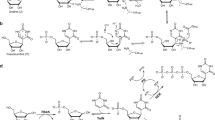
Previous research has identified ribose aminooxazoline as a potential intermediate in the prebiotic synthesis of the pyrimidine nucleotides with remarkable properties. It crystallizes spontaneously from reaction mixtures, with an enhanced enantiomeric excess if initially enantioenriched, which suggests that reservoirs of this compound might have accumulated on the early Earth in an optically pure form. Ribose aminooxazoline can be converted efficiently into α-ribocytidine by way of 2,2′-anhydroribocytidine, although anomerization to β-ribocytidine by ultraviolet irradiation is extremely inefficient. Our previous work demonstrated the synthesis of pyrimidine β-ribonucleotides, but at the cost of ignoring ribose aminooxazoline, using arabinose aminooxazoline instead. Here we describe a long-sought route through ribose aminooxazoline to the pyrimidine β-ribonucleosides and their phosphate derivatives that involves an extraordinarily efficient photoanomerization of α-2-thioribocytidine. In addition to the canonical nucleosides, our synthesis accesses β-2-thioribouridine, a modified nucleoside found in transfer RNA that enables both faster and more-accurate nucleic acid template-copying chemistry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sanchez, R. A. & Orgel, L. E. Studies in prebiotic synthesis. V. Synthesis and photoanomerization of pyrimidine nucleosides. J. Mol. Biol. 47, 531–543 (1970).
Powner, M. W. et al. On the prebiotic synthesis of ribonucleotides: photoanomerisation of cytosine nucleosides and nucleotides revisited. ChemBioChem 8, 1170–1179 (2007).
Powner, M. W., Gerland, B. & Sutherland, J. D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459, 239–242 (2009).
Anastasi, C., Crowe, M. A., Powner, M. W. & Sutherland, J. D. Direct assembly of nucleoside precursors from two- and three-carbon units. Angew. Chem. Int. Ed. 45, 6176–6179 (2006).
Powner, M. W. & Sutherland, J. D. Phosphate-mediated interconversion of ribo- and arabino-configured prebiotic nucleotide intermediates. Angew. Chem. Int. Ed. 49, 4641–4643 (2010).
Ritson, D. & Sutherland, J. D. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nat. Chem. 4, 895–899 (2012).
Ritson, D. J. & Sutherland, J. D. Synthesis of aldehydic ribonucleotide and amino acid precursors by photoredox chemistry. Angew. Chem. Int. Ed. 52, 5845–5847 (2013).
Kawaguchi, T. et al. Enzymatic reactivity and anti-tumor activity of 1-(β-D-arabinofuranosyl)-2-thiocytosine derivatives. Chem. Pharm. Bull. 48, 454–457 (2000).
Frick, L., Mac Neela, J. P. & Wolfenden, R. Transition state stabilization by deaminases: rates of nonenzymatic hydrolysis of adenosine and cytidine. Bioorg. Chem. 15, 100–108 (1987).
Schoffstall, A. M. Prebiotic phosphorylation of nucleosides in formamide. Orig. Life 7, 399–412 (1976).
Lohrmann, R. &. Orgel, L. E. Urea–inorganic phosphate mixtures as prebiotic phosphorylating agents. Science 171, 490–494 (1971).
Saenger, W. Principles of Nucleic Acid Structure (Springer, 1984).
Brown, D. J. & Jacobsen, N. W. 612. Pyrimidine reactions. Part IV. The methylation of 2,4- and 4,5-diaminopyrimidine and related compounds. J. Chem. Soc. 3172–3179 (1962).
Szabla, R. et al. Excited-state hydrogen atom abstraction initiates the photochemistry of β-2′-deoxycytidine. Chem. Sci. 6, 2035–2043 (2015).
Mai, S., Marquetand, P. & González, L. Intersystem crossing pathways in the noncanonical nucleobase 2-thiouracil: a time-dependent picture. J. Phys. Chem. Lett. 7, 1978–1983 (2016).
Martínez-Fernández, L., Corral, I., Granucci, G. & Persico, M. Competing ultrafast intersystem crossing and internal conversion: a time resolved picture for the deactivation of 6-thioguanine. Chem. Sci. 5, 1336–1347 (2014).
Pollum, M. & Crespo-Hernández, C. E. Communication: the dark singlet state as a doorway state in the ultrafast and efficient intersystem crossing dynamics in 2-thiothymine and 2 thiouracil. J. Chem. Phys. 140, 071101 (2014).
Taras-Goślińska, K., Burdziński, G. & Wenska, G. Relaxation of the T1 excited state of 2-thiothymine, its riboside and deoxyriboside-enhanced nonradiative decay rate induced by sugar substituent. J. Photochem. Photobiol. Chem. 275, 89–95 (2014).
Gobbo, J. P. & Borin, A. C. 2-Thiouracil deactivation pathways and triplet states population. Comput. Theor. Chem. 1040, 195–201 (2014).
Mai, S., Marquetand, P. & González, L. A static picture of the relaxation and intersystem crossing mechanisms of photoexcited 2-thiouracil. J. Phys. Chem. A 119, 9524–9533 (2015).
Besley, N. A. & Hirst, J. D. Ab initio study of the electronic spectrum of formamide with explicit solvent. J. Am. Chem. Soc. 121, 8559–8566 (1999).
Joyce, G. F., Schwartz, A. W., Miller, S. L. & Orgel, L. E. The case for an ancestral genetic system involving simple analogues of the nucleotides. Proc. Natl Acad. Sci. USA 84, 4398–4402 (1987).
Grosjean, H., de Crécy-Lagard, V. & Marck, C. Deciphering synonymous codons in the three domains of life: co-evolution with specific tRNA modification enzymes. FEBS Lett. 584, 252–264 (2010).
Heuberger, B. D., Pal, A., Del Frate, F., Topkar, V. V. & Szostak, J. W. Replacing uridine with 2-thiouridine enhances the rate and fidelity of nonenzymatic RNA primer extension. J. Am. Chem. Soc. 137, 2769–2775 (2015).
Acknowledgements
This work was supported by the Medical Research Council (no. MC_UP_A024_1009), a grant from the Simons Foundation (no. 290362 to J.D.S.), grant 14-12010S from the Grant Agency of the Czech Republic and by the project CEITEC 2020 (LQ1601) with financial support from the Ministry of Education, Youth and Sports of the Czech Republic under the National Sustainability Programme II. Support from a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry of Wrocław University of Technology is gratefully acknowledged. Theoretical calculations were partly performed at the Wrocław Center for Networking and Supercomputing and Interdisciplinary Centre for Mathematical and Computational Modelling in Warsaw.
Author information
Authors and Affiliations
Contributions
J.D.S. supervised the experimental research, and J.X., M.T. and C.J.M. performed the experiments. J.E.S., J.S. and R.W.G. oversaw the theoretical work, which was carried out by R.S. All the authors contributed intellectually as the project unfolded. J.D.S. wrote most of the paper and J.X., M.T., C.J.M. and R.S. further contributed and assembled the Supplementary Information.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 41155 kb)
Rights and permissions
About this article
Cite this article
Xu, J., Tsanakopoulou, M., Magnani, C. et al. A prebiotically plausible synthesis of pyrimidine β-ribonucleosides and their phosphate derivatives involving photoanomerization. Nature Chem 9, 303–309 (2017). https://doi.org/10.1038/nchem.2664
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2664
This article is cited by
-
Chirality-induced avalanche magnetization of magnetite by an RNA precursor
Nature Communications (2023)
-
Thiophosphate photochemistry enables prebiotic access to sugars and terpenoid precursors
Nature Chemistry (2023)
-
Prebiotic synthesis and triphosphorylation of 3′-amino-TNA nucleosides
Nature Chemistry (2022)
-
Ariel – a window to the origin of life on early earth?
Experimental Astronomy (2022)
-
2,6-diaminopurine promotes repair of DNA lesions under prebiotic conditions
Nature Communications (2021)