Abstract
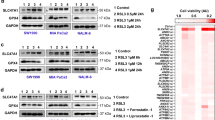
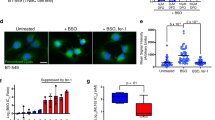
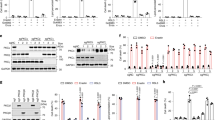
Ferroptosis is a form of regulated necrotic cell death controlled by glutathione peroxidase 4 (GPX4). At present, mechanisms that could predict sensitivity and/or resistance and that may be exploited to modulate ferroptosis are needed. We applied two independent approaches—a genome-wide CRISPR-based genetic screen and microarray analysis of ferroptosis-resistant cell lines—to uncover acyl-CoA synthetase long-chain family member 4 (ACSL4) as an essential component for ferroptosis execution. Specifically, Gpx4–Acsl4 double-knockout cells showed marked resistance to ferroptosis. Mechanistically, ACSL4 enriched cellular membranes with long polyunsaturated ω6 fatty acids. Moreover, ACSL4 was preferentially expressed in a panel of basal-like breast cancer cell lines and predicted their sensitivity to ferroptosis. Pharmacological targeting of ACSL4 with thiazolidinediones, a class of antidiabetic compound, ameliorated tissue demise in a mouse model of ferroptosis, suggesting that ACSL4 inhibition is a viable therapeutic approach to preventing ferroptosis-related diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Conrad, M., Angeli, J.P., Vandenabeele, P. & Stockwell, B.R. Regulated necrosis: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 15, 348–366 (2016).
Friedmann Angeli, J.P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Matsushita, M. et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212, 555–568 (2015).
Yang, W.S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Linkermann, A. et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl. Acad. Sci. USA 111, 16836–16841 (2014).
Jiang, L. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57–62 (2015).
Dolma, S., Lessnick, S.L., Hahn, W.C. & Stockwell, B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3, 285–296 (2003).
Dixon, S.J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Ishii, T., Sugita, Y. & Bannai, S. Regulation of glutathione levels in mouse spleen lymphocytes by transport of cysteine. J. Cell. Physiol. 133, 330–336 (1987).
Ursini, F., Maiorino, M., Valente, M., Ferri, L. & Gregolin, C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim. Biophys. Acta 710, 197–211 (1982).
Yang, W.S. & Stockwell, B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 15, 234–245 (2008).
Dixon, S.J. et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3, e02523 (2014).
Louandre, C. et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int. J. Cancer 133, 1732–1742 (2013).
Hayano, M., Yang, W.S., Corn, C.K., Pagano, N.C. & Stockwell, B.R. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 23, 270–278 (2016).
Seiler, A. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8, 237–248 (2008).
Koike-Yusa, H., Li, Y., Tan, E.P., Velasco-Herrera, Mdel.C. & Yusa, K. Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat. Biotechnol. 32, 267–273 (2014).
Wortmann, M. et al. Combined deficiency in glutathione peroxidase 4 and vitamin E causes multiorgan thrombus formation and early death in mice. Circ. Res. 113, 408–417 (2013).
Canli, Ö. et al. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood 127, 139–148 (2016).
Soupene, E., Fyrst, H. & Kuypers, F.A. Mammalian acyl-CoA:lysophosphatidylcholine acyltransferase enzymes. Proc. Natl. Acad. Sci. USA 105, 88–93 (2008).
Yamanaka, K. et al. A novel fluorescent probe with high sensitivity and selective detection of lipid hydroperoxides in cells. RSC Advances 2, 7894–7900 (2012).
Dixon, S.J. et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem. Biol. 10, 1604–1609 (2015).
Yin, H., Xu, L. & Porter, N.A. Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 111, 5944–5972 (2011).
Pratt, D.A., Mills, J.H. & Porter, N.A. Theoretical calculations of carbon-oxygen bond dissociation enthalpies of peroxyl radicals formed in the autoxidation of lipids. J. Am. Chem. Soc. 125, 5801–5810 (2003).
Kagan, V.E. Oxidized arachidonic and adrenic phosphatidylethanolamines navigate cells to ferroptosis. Nat. Chem. Biol. http://dx.doi.org/nchembio.2238 (2016).
Timmerman, L.A. et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 24, 450–465 (2013).
Kim, J.H., Lewin, T.M. & Coleman, R.A. Expression and characterization of recombinant rat Acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J. Biol. Chem. 276, 24667–24673 (2001).
Gale, E.A. Lessons from the glitazones: a story of drug development. Lancet 357, 1870–1875 (2001).
Küch, E.M. et al. Differentially localized acyl-CoA synthetase 4 isoenzymes mediate the metabolic channeling of fatty acids towards phosphatidylinositol. Biochim. Biophys. Acta 1841, 227–239 (2014).
Van Horn, C.G. et al. Characterization of recombinant long-chain rat acyl-CoA synthetase isoforms 3 and 6: identification of a novel variant of isoform 6. Biochemistry 44, 1635–1642 (2005).
Brash, A.R. Arachidonic acid as a bioactive molecule. J. Clin. Invest. 107, 1339–1345 (2001).
Orlando, U.D. et al. Acyl-CoA synthetase-4, a new regulator of mTOR and a potential therapeutic target for enhanced estrogen receptor function in receptor-positive and -negative breast cancer. Oncotarget 6, 42632–42650 2015).
Wu, X. et al. ACSL4 promotes prostate cancer growth, invasion and hormonal resistance. Oncotarget 6, 44849–44863 (2015).
Wu, X. et al. Long chain fatty acyl-CoA synthetase 4 is a biomarker for and mediator of hormone resistance in human breast cancer. PLoS One 8, e77060 (2013).
Monaco, M.E. et al. Expression of long-chain fatty acyl-CoA synthetase 4 in breast and prostate cancers is associated with sex steroid hormone receptor negativity. Transl. Oncol. 3, 91–98 (2010).
Hudis, C.A. & Gianni, L. Triple-negative breast cancer: an unmet medical need. Oncologist 16 (Suppl. 1), 1–11 (2011).
Jin, J. et al. Neuroprotective effects of PPAR-γ agonist rosiglitazone in N171-82Q mouse model of Huntington's disease. J. Neurochem. 125, 410–419 (2013).
Heneka, M.T., Fink, A. & Doblhammer, G. Effect of pioglitazone medication on the incidence of dementia. Ann. Neurol. 78, 284–294 (2015).
Belfort, R. et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 355, 2297–2307 (2006).
Aithal, G.P. et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 135, 1176–1184 (2008).
Han, L. et al. Rosiglitazone promotes white matter integrity and long-term functional recovery after focal cerebral ischemia. Stroke 46, 2628–2636 (2015).
Culman, J. et al. Treatment of rats with pioglitazone in the reperfusion phase of focal cerebral ischemia: a preclinical stroke trial. Exp. Neurol. 238, 243–253 (2012).
Rennings, A.J. et al. Rosiglitazone reduces ischaemia-reperfusion injury in patients with the metabolic syndrome. Eur. Heart J. 31, 983 (2010).
Wu, J.S. et al. Ligand-activated peroxisome proliferator-activated receptor-gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3 epsilon upregulation. Circulation 119, 1124–1134 (2009).
Kuboki, S. et al. Peroxisome proliferator-activated receptor-γ protects against hepatic ischemia/reperfusion injury in mice. Hepatology 47, 215–224 (2008).
Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem. 27, 502–522 (1969).
Bannai, S. & Ishii, T. Transport of cystine and cysteine and cell growth in cultured human diploid fibroblasts: effect of glutamate and homocysteate. J. Cell. Physiol. 112, 265–272 (1982).
Roveri, A., Maiorino, M. & Ursini, F. Enzymatic and immunological measurements of soluble and membrane-bound phospholipid-hydroperoxide glutathione peroxidase. Methods Enzymol. 233, 202–212 (1994).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Brinkman, E.K., Chen, T., Amendola, M. & van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42, e168 (2014).
Mannes, A.M., Seiler, A., Bosello, V., Maiorino, M. & Conrad, M. Cysteine mutant of mammalian GPx4 rescues cell death induced by disruption of the wild-type selenoenzyme. FASEB J. 25, 2135–2144 (2011).
Haack, T.B. et al. ELAC2 mutations cause a mitochondrial RNA processing defect associated with hypertrophic cardiomyopathy. Am. J. Hum. Genet. 93, 211–223 (2013).
Bornkamm, G.W. et al. Stringent doxycycline-dependent control of gene activities using an episomal one-vector system. Nucleic Acids Res. 33, e137 (2005).
Acknowledgements
We would like to thank B.R. Stockwell (Columbia University, New York, USA) for providing RSL3 and F. Ursini and M. Maiorino (Universitàdegli Studi di Padova, Padua, Italy) for phosphatidylcholine hydroperoxide. We would also like to thank A. Berns (the Netherlands Cancer Institute) for providing the ROSA26-CreERT2 mouse line. SK-BR-3 cells were a gift from G. Multhoff (Technical University Munich). This work was in part supported by grants from the Deutsche Forschungsgemeinschaft (DFG) CO 291/2-3 and CO 291/5-1 to M.C., a fellowship from the Japan Society for the Promotion of Science (JSPS) to S.K., the Human Frontier Science Program (HFSP) RGP0013 to M.C. and V.E.K., the German Bundesministerium für Bildung und Forschung (BMBF) through the European E-Rare Network for mitochondrial disorders GENOMIT (01GM1603) to H.P., the National Institute of Health (NIH) (P01HL114453, U19AI068021, NS076511, NS061817, ES020693) to V.E.K. and the Bavarian Ministry of Economic Affairs (m4 Award) to J.A.S. and M.C.
Author information
Authors and Affiliations
Contributions
J.P.F.A., S.D. and M.C. conceived the study. M.A. and A.W. carried out and analyzed electron microscopy studies. Y.Y.T., G.M., F.Q., H.B. and V.E.K. performed oxi-lipidomics analysis and data interpretation. M.I., J.B., H.P. and D.T. conducted microarray analysis, deep-sequencing and analysis. S.D., B.P., E.P., S.K., I.I. and J.P.F.A. performed in vitro and in vivo experiments. J.F., C.H.S. and W.W. provided reagents and participated in the discussion. S.D., J.A.S., J.P.F.A. and M.C. performed evaluation and interpretation of the in vitro data. J.P.F.A. and M.C. wrote the paper. All authors read and agreed on the content of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results and Supplementary Figures 1–6. (PDF 13257 kb)
Supplementary Table 1
Statistical analysis of expression profiling of ferroptosis-resistant cells. (XLSX 38949 kb)
Supplementary Table 2
Single gRNA counts after RSL3 and erastin selections. (XLSX 8139 kb)
Supplementary Table 3
Gene list of expression profiling of ferroptosis-resistant cells. (XLSX 9555 kb)
Rights and permissions
About this article
Cite this article
Doll, S., Proneth, B., Tyurina, Y. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13, 91–98 (2017). https://doi.org/10.1038/nchembio.2239
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2239
This article is cited by
-
Harnessing ferroptosis for enhanced sarcoma treatment: mechanisms, progress and prospects
Experimental Hematology & Oncology (2024)
-
Ferroptosis in early brain injury after subarachnoid hemorrhage: review of literature
Chinese Neurosurgical Journal (2024)
-
Ferroptosis contributing to cardiomyocyte injury induced by silica nanoparticles via miR-125b-2-3p/HO-1 signaling
Particle and Fibre Toxicology (2024)
-
Modulating ferroptosis sensitivity: environmental and cellular targets within the tumor microenvironment
Journal of Experimental & Clinical Cancer Research (2024)
-
Ferroptosis: a promising candidate for exosome-mediated regulation in different diseases
Cell Communication and Signaling (2024)