Abstract
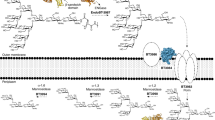
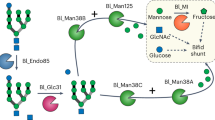
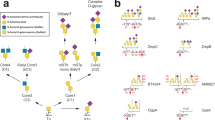
Colonic bacteria, exemplified by Bacteroides thetaiotaomicron, play a key role in maintaining human health by harnessing large families of glycoside hydrolases (GHs) to exploit dietary polysaccharides and host glycans as nutrients. Such GH family expansion is exemplified by the 23 family GH92 glycosidases encoded by the B. thetaiotaomicron genome. Here we show that these are α-mannosidases that act via a single displacement mechanism to utilize host N-glycans. The three-dimensional structure of two GH92 mannosidases defines a family of two-domain proteins in which the catalytic center is located at the domain interface, providing acid (glutamate) and base (aspartate) assistance to hydrolysis in a Ca2+-dependent manner. The three-dimensional structures of the GH92s in complex with inhibitors provide insight into the specificity, mechanism and conformational itinerary of catalysis. Ca2+ plays a key catalytic role in helping distort the mannoside away from its ground-state 4C1 chair conformation toward the transition state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ley, R.E., Peterson, D.A. & Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124, 837–848 (2006).
Backhed, F., Ley, R.E., Sonnenburg, J.L., Peterson, D.A. & Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005).
Turnbaugh, P.J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Wen, L. et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455, 1109–1113 (2008).
Mazmanian, S.K., Round, J.L. & Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625 (2008).
Kim, Y.S. & Milner, J.A. Dietary modulation of colon cancer risk. J. Nutr. 137, 2576S–2579S (2007).
Cerdeno-Tarraga, A.M. et al. Extensive DNA inversions in the B. fragilis genome control variable gene expression. Science 307, 1463–1465 (2005).
Xu, J. et al. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 299, 2074–2076 (2003).
Xu, J. et al. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol. 5, e156 (2007).
Gill, S.R. et al. Metagenomic analysis of the human distal gut microbiome. Science 312, 1355–1359 (2006).
Turnbaugh, P.J. et al. The human microbiome project. Nature 449, 804–810 (2007).
Maruyama, Y., Nakajima, T. & Ichishima, E. A 1,2-alpha-D-mannosidase from a Bacillus sp.: purification, characterization, and mode of action. Carbohydr. Res. 251, 89–98 (1994).
Davies, G.J., Wilson, K.S. & Henrissat, B. Nomenclature for sugar-binding subsites in glycosyl hydrolases. Biochem. J. 321, 557–559 (1997).
Vocadlo, D. & Davies, G.J. Mechanistic insights into glycosidase chemistry. Curr. Opin. Chem. Biol. 12, 539–555 (2008).
Krissinel, E. & Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 60, 2256–2268 (2004).
Heikinheimo, P. et al. The structure of bovine lysosomal alpha-mannosidase suggests a novel mechanism for low-pH activation. J. Mol. Biol. 327, 631–644 (2003).
Gibson, R.P. et al. Molecular basis for trehalase inhibition revealed by the structure of trehalase in complex with potent inhibitors. Angew. Chem. Int. Ed. 46, 4115–4119 (2007).
Nurizzo, D., Nagy, T., Gilbert, H.J. & Davies, G.J. The structural basis for catalysis and specificity of the Pseudomonas cellulosa α-glucuronidase, GlcA67A. Structure 10, 547–556 (2002).
Damude, H.G., Withers, S.G., Kilburn, D.G., Miller, R.C. Jr & Warren, R.A.J. Site-directed mutation of the putative catalytic residues of endoglucanase CenA from Cellulomonas fimi. Biochemistry 34, 2220–2224 (1995).
Davies, G.J., Ducros, V.M.A., Varrot, A. & Zechel, D.L. Mapping the conformational itinerary of β-glycosidases by X-ray crystallography. Biochem. Soc. Trans. 31, 523–527 (2003).
Vallee, F., Karaveg, K., Herscovics, A., Moremen, K.W. & Howell, P.L. Structural basis for catalysis and inhibition of N-glycan processing class I alpha 1,2-mannosidases. J. Biol. Chem. 275, 41287–41298 (2000).
Numao, S., Kuntz, D.A., Withers, S.G. & Rose, D.R. Insights into the mechanism of Drosophila melanogaster Golgi alpha-mannosidase II through the structural analysis of covalent reaction intermediates. J. Biol. Chem. 278, 48074–48083 (2003).
Karaveg, K. et al. Mechanism of class 1 (Glycosylhydrolase family 47) alpha-mannosidases involved in N-glycan processing and endoplasmic reticulum quality control. J. Biol. Chem. 280, 16197–16207 (2005).
Ducros, V. et al. Substrate distortion by a β-mannanase: snapshots of the Michaelis and covalent intermediate complexes suggest a B2,5 conformation for the transition-state. Angew. Chem. Int. Ed. 41, 2824–2827 (2002).
Tailford, L.E. et al. Structural and biochemical evidence for a boat-like transition state in β-mannosidases. Nat. Chem. Biol. 4, 306–312 (2008).
Offen, W.A., Zechel, D.L., Withers, S.G., Gilbert, H.J. & Davies, G.J. Structure of the Michaelis complex of β−mannosidase, Man2A, provides insight into the conformational itinerary of mannoside hydrolysis. Chem. Commun. (Camb.) 2484–2486 (2009).
Whitfield, D.M. DFT studies of the ionization of alpha and beta glycopyranosyl donors. Carbohydr. Res. 342, 1726–1740 (2007).
Terinek, M. & Vasella, A. Synthesis and evaluation of two mannosamine-derived lactone-type inhibitors of snail beta-mannosidase. Tetrahedron Asymmetry 16, 449–469 (2005).
Kayakiri, H. et al. Structure of kifunensine, a new immunomodulator isolated from an actinomycete. J. Org. Chem. 54, 4015–4016 (1989).
Crich, D. & Chandrasekera, N.S. Mechanism of 4,6–0-benzylidene-directed beta-mannosylation as determined by alpha-deuterium kinetic isotope effects. Angew. Chem. Int. Ed. 43, 5386–5389 (2004).
Bjursell, M.K., Martens, E.C. & Gordon, J.I. Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroides thetaiotaomicron, to the suckling period. J. Biol. Chem. 281, 36269–36279 (2006).
Martens, E.C., Chiang, H.C. & Gordon, J.I. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 4, 447–457 (2008).
Ito, Y. & Ogawa, T. A novel-approach to the stereoselective synthesis of beta-mannosides. Angew. Chem. Int. Edn Engl. 33, 1765–1767 (1994).
Gridley, J.J. & Osborn, H.M.I. Recent advances in the construction of b-D-mannose and b-D-mannosamine linkages. J. Chem. Soc. Perkin Trans. 1 10, 1471–1491 (2000).
Granovsky, M. et al. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med. 6, 306–312 (2000).
Kuntz, D., Zhong, W., Guo, J., Rose, D. & Boons, G. The molecular basis of inhibition of Golgi alpha-mannosidase II by mannostatin A. ChemBioChem 10, 268–277 (2009).
Hogg, D. et al. Crystal structure of mannanase 26A from Pseudomonas cellulosa and analysis of residues involved in substrate binding. J. Biol. Chem. 276, 31186–31192 (2001).
Bolam, D. et al. Mannanase A from Pseudomonas fluorescens ssp. cellulosa is a retaining glycosyl hydrolase in which E212 and E320 are the putative catalytic residues. Biochemistry 35, 16195–16204 (1996).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Terwilliger, T.C. & Berendzen, J. Automated MAD and MIR structure solution. Acta Crystallogr. D Biol. Crystallogr. 55, 849–861 (1999).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Acknowledgements
We thank S. Withers (University of British Columbia) for compound 9. The Biotechnology and Biological Sciences Research Council of the UK is thanked for funding. M.D.L.S. is a European Molecular Biology Laboratory long-term fellowship holder, and G.J.D. is a Royal Society/Wolfson Research Merit Award recipient. C.D. was supported by a Marie Curie European Reintegration grant within the 7th European Community Framework Programme. Y.Z. is supported by the Department for Innovation, Universities and Skills (DIUS), UK, Newcastle University and the Chinese Scholarship Council. S.J.W. thanks the Australian Research Council for funding support. K.W.M. acknowledges the support of US National Institutes of Health grants GM047533, RR005351 and DK075322. S.C. is supported by a postdoctoral fellowship from the Agence Nationale de Recherche sur le Sida et les Hépatities Virales (ANRS). A.S. is supported by Centre National de la Recherche Scientifique.
Author information
Authors and Affiliations
Contributions
H.J.G. and G.J.D. designed the experiments. Y.Z., M.D.L.S., A.J.T., C.D., N.S., Y.X. and K.W.M. performed or supervised molecular biology, kinetics, glycan analysis and X-ray crystallography. S.C., A.S., Z.D. and S.J.W. synthesized ligands. H.J.G., G.J.D. and S.J.W., aided by the other authors, wrote the paper.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–6 (PDF 1893 kb)
Rights and permissions
About this article
Cite this article
Zhu, Y., Suits, M., Thompson, A. et al. Mechanistic insights into a Ca2+-dependent family of α-mannosidases in a human gut symbiont. Nat Chem Biol 6, 125–132 (2010). https://doi.org/10.1038/nchembio.278
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.278
This article is cited by
-
Carbohydrates and carbohydrate degradation gene abundance and transcription in Atlantic waters of the Arctic
ISME Communications (2023)
-
Design and directed evolution of noncanonical β-stereoselective metalloglycosidases
Nature Communications (2022)
-
Glycoside hydrolase from the GH76 family indicates that marine Salegentibacter sp. Hel_I_6 consumes alpha-mannan from fungi
The ISME Journal (2022)
-
A mucin-responsive hybrid two-component system controls Bacteroides thetaiotaomicron colonization and gut homeostasis
Journal of Microbiology (2022)
-
Quantifying fluorescent glycan uptake to elucidate strain-level variability in foraging behaviors of rumen bacteria
Microbiome (2021)