Abstract
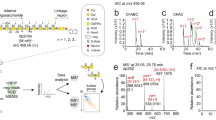
Proteoglycans are complex glycoconjugates that regulate critical biological pathways in all higher organisms. Bikunin, the simplest proteoglycan, with a single glycosaminoglycan chain, is a serine protease inhibitor used to treat acute pancreatitis. Unlike nucleic acids and proteins, whose synthesis is template driven, Golgi-synthesized glycosaminoglycans are not believed to have predictable or deterministic sequences. Bikunin peptidoglycosaminoglycans were prepared and fractionated to obtain a collection of size-similar and charge-similar chains. Fourier transform mass spectral analysis identified a small number of parent molecular ions corresponding to monocompositional peptidoglycosaminoglycans. Fragmentation using collision-induced dissociation unexpectedly afforded a single sequence for each monocompositional parent ion, unequivocally demonstrating the presence of a defined sequence. The biosynthetic pathway common to all proteoglycans suggests that even more structurally complex proteoglycans, such as heparan sulfate, may have defined sequences, requiring a readjustment in the understanding of information storage in complex glycans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Feero, W.G., Guttmacher, A.E. & Collins, F.S. Genomic medicine–an updated primer. N. Engl. J. Med. 362, 2001–2011 (2010).
Ly, M., Laremore, T.N. & Linhardt, R.J. Proteoglycomics: recent progress and future challenges. OMICS 14, 389–399 (2010)10.1089/omi.2009.0123.
Kreuger, J., Spillmann, D., Li, J. & Lindahl, U. Interactions between heparan sulfate and proteins: the concept of specificity. J. Cell Biol. 174, 323–327 (2006).
Ball, S. et al. From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell 86, 349–352 (1996).
Perez, S. & Mazeau, K. Conformations, structures, and morphologies of celluloses. in Polysaccharides: Structural diversity and functional versatility 2nd edn (ed. Dumitiu, S.) 41–68 (Marcel Dekker, 1998).
Esko, J.D. & Selleck, S.B. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 71, 435–471 (2002).
Silbert, J.E. & Sugumaran, G. Biosynthesis of chondroitin/dermatan sulfate. IUBMB Life 54, 177–186 (2002).
Nairn, A.V. et al. Glycomics of proteoglycan biosynthesis in murine embryonic stem cell differentiation. J. Proteome Res. 6, 4374–4387 (2007)10.1021/pr070446f.
Couchman, J.R. Transmembrane signaling proteoglycans. Annu. Rev. Cell Dev. Biol. 26, 89–114 (2010).
Linhardt, R.J. & Toida, T. Role of glycosaminoglycans in cellular communication. Acc. Chem. Res. 37, 431–438 (2004)10.1021/ar030138x.
Atha, D.H., Lormeau, J.C., Petitou, M., Rosenberg, R.D. & Choay, J. Contribution of 3-O- and 6-O-sulfated glucosamine residues in the heparin-induced conformational change in antithrombin III. Biochemistry 26, 6454–6461 (1987).
Petitou, M. & van Boeckel, C.A. A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next? Angew. Chem. Int. Edn Engl. 43, 3118–3133 (2004).
Fries, E. & Blom, A.M. Bikunin–not just a plasma proteinase inhibitor. Int. J. Biochem. Cell Biol. 32, 125–137 (2000).
Fries, E. & Kaczmarczyk, A. Inter-alpha-inhibitor, hyaluronan and inflammation. Acta Biochim. Pol. 50, 735–742 (2003).
Zhuo, L., Salustri, A. & Kimata, K. A physiological function of serum proteoglycan bikunin: the chondroitin sulfate moiety plays a central role. Glycoconj. J. 19, 241–247 (2002)10.1023/A:1025331929373.
Michalski, C. et al. Preparation and properties of a therapeutic inter-alpha-trypsin inhibitor concentrate from human plasma. Vox Sang. 67, 329–336 (1994)10.1111/j.1423-0410.1994.tb01269.x.
Enghild, J.J. et al. Chondroitin 4-sulfate covalently cross-links the chains of the human blood protein pre-alpha-inhibitor. J. Biol. Chem. 266, 747–751 (1991).
Morelle, W. et al. Chondroitin sulphate covalently cross-links the three polypeptide chains of inter-alpha-trypsin inhibitor. Eur. J. Biochem. 221, 881–888 (1994).
Zhuo, L., Hascall, V.C. & Kimata, K. Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J. Biol. Chem. 279, 38079–38082 (2004).
Enghild, J.J. et al. Organization of the inter-alpha-inhibitor heavy chains on the chondroitin sulfate originating from Ser(10) of bikunin: posttranslational modification of IalphaI-derived bikunin. Biochemistry 38, 11804–11813 (1999).
Josic, D. et al. Proteomic characterization of inter-alpha inhibitor proteins from human plasma. Proteomics 6, 2874–2885 (2006).
Delaria, K.A. et al. Characterization of placental bikunin, a novel human serine protease inhibitor. J. Biol. Chem. 272, 12209–12214 (1997)10.1074/jbc.272.18.12209.
Chi, L. et al. Structural analysis of bikunin glycosaminoglycan. J. Am. Chem. Soc. 130, 2617–2625 (2008)10.1021/ja0778500.
Conrad, H.E. Beta-elimination for release of O-linked glycosaminoglycans from proteoglycans. Curr. Protoc. Mol. Biol. 17, 15.1–15.3 (2001)10.1002/0471142727.mb1715as31.
Venkataraman, G., Shriver, Z., Raman, R. & Sasisekharan, R. Sequencing complex polysaccharides. Science 286, 537–542 (1999).
Laremore, T.N. et al. Domain structure elucidation of human decorin glycosaminoglycans. Biochem. J. 431, 199–205 (2010)10.1042/BJ20100788.
Wolff, J.J., Amster, I.J., Chi, L. & Linhardt, R.J. Electron detachment dissociation of glycosaminoglycan tetrasaccharides. J. Am. Soc. Mass Spectrom. 18, 234–244 (2007)10.1016/j.jasms.2006.09.020.
Wolff, J.J., Laremore, T.N., Aslam, H., Linhardt, R.J. & Amster, I.J. Electron-induced dissociation of glycosaminoglycan tetrasaccharides. J. Am. Soc. Mass Spectrom. 19, 1449–1458 (2008)10.1016/j.jasms.2008.06.024.
Wolff, J.J. et al. Negative electron transfer dissociation of glycosaminoglycans. Anal. Chem. 82, 3460–3466 (2010)10.1021/ac100554a.
Turnbull, J.E., Hopwood, J.J. & Gallagher, J.T. A strategy for rapid sequencing of heparan sulfate and heparin saccharides. Proc. Natl. Acad. Sci. USA 96, 2698–2703 (1999).
Merry, C.L.R., Lyon, M., Deakin, J.A., Hopwood, J.J. & Gallagher, J.T. Highly sensitive sequencing of the sulfated domains of heparan sulfate. J. Biol. Chem. 274, 18455–18462 (1999).
Turnbull, J.E. & Gallager, J.T. Sequence analysis of heparan sulphate indicates defined location of N-sulphated glucosamine and iduronate 2-sulphate residues proximal to the protein linkage region. Biochem. J. 277, 297–303 (1991).
Liu, J., Desai, U.R., Han, X.-J., Toida, T. & Linhardt, R.J. Strategy for the sequence analysis of heparin. Glycobiology 5, 765–774 (1995)10.1093/glycob/5.8.765.
Zaia, J., Li, X.Q., Chan, S.Y. & Costello, C.E. Tandem mass spectrometric strategies for determination of sulfation positions and uronic acid epimerization in chondroitin sulfate oligosaccharides. J. Am. Soc. Mass Spectrom. 14, 1270–1281 (2003)10.1016/S1044-0305(03)00541-5.
McClellan, J.E., Costello, C.E., O'Connor, P.B. & Zaia, J. Influence of charge state on product ion mass spectra and the determination of 4S/6S sulfation sequence of chondroitin sulfate oligosaccharides. Anal. Chem. 74, 3760–3771 (2002).
Hitchcock, A.M., Yates, K.E., Costello, C.E. & Zaia, J. Comparative glycomics of connective tissue glycosaminoglycans. Proteomics 8, 1384–1397 (2008)10.1002/pmic.200700787.
Toyoda, H., Kobayashi, S., Sakamoto, S., Toida, T. & Imanari, T. Structural analysis of a low-sulfated chondroitin sulfate chain in human urinary trypsin inhibitor. Biol. Pharm. Bull. 16, 945–947 (1993).
Yamada, S. et al. The sulphated carbohydrate-protein linkage region isolated from chondroitin 4-sulphate chains of inter-alpha-trypsin inhibitor in human plasma. Glycobiology 5, 335–341 (1995).
Laremore, T.N., Ly, M., Solakyildirim, K., Zagorevski, D.V. & Linhardt, R.J. High-resolution preparative separation of glycosaminoglycan oligosaccharides by polyacrylamide gel electrophoresis. Anal. Biochem. 401, 236–241 (2010)10.1016/j.ab.2010.03.004.
Ly, M. et al. Analysis of E. coli K5 capsular polysaccharide heparosan. Anal. Bioanal. Chem. 399, 737–745 (2011)10.1007/s00216-010-3679-7.
Wolff, J.J., Laremore, T.N., Busch, A.M., Linhardt, R.J. & Amster, I.J. Influence of charge state and sodium cationization on the electron detachment dissociation and infrared multiphoton dissociation of glycosaminoglycan oligosaccharides. J. Am. Soc. Mass Spectrom. 19, 790–798 (2008)10.1016/j.jasms.2008.03.010.
Laremore, T.N., Leach, F.E. III, Amster, I.J. & Linhardt, R.J. Electrospray ionization Fourier transform mass spectrometric analysis of intact bikunin glycosaminoglycan from normal human plasma. Int. J. Mass. Spectrom. 305, 109–115 (2011)10.1016/j.ijms.2010.09.020.
Ly, M. Glycosaminoglycan Sequencing of Proteoglycan. PhD thesis, Rensselaer Polytechnic Institute (2011).
Gu, K., Liu, J., Pervin, A. & Linhardt, R.J. Comparison of the activity of two chondroitin AC lyases on dermatan sulfate. Carbohydr. Res. 244, 369–377 (1993).
Zaia, J. Principles of mass spectrometry of glycosaminoglycans. J. Biomacromol. Mass. Spec. 1, 3–36 (2005).
Gunay, N.S., Tadano-Aritomi, K., Toida, T., Ishizuka, I. & Linhardt, R.J. Evaluation of counterions for electrospray ionization mass spectral analysis of a highly sulfated carbohydrate, sucrose octasulfate. Anal. Chem. 75, 3226–3231 (2003)10.1021/ac034053l.
Capon, C., Mizon, C., Lemoine, J., Rodié-Talbère, P. & Mizon, J. In acute inflammation, the chondroitin-4 sulphate carried by bikunin is not only longer; it is also undersulphated. Biochimie 85, 101–107 (2003)10.1016/S0300-9084(03)00066-X.
Bitter, T. & Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 4, 330–334 (1962).
Ceroni, A. et al. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 7, 1650–1659 (2008).
Domon, B. & Costello, C. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 5, 397–409 (1988).
Acknowledgements
The authors thank D. Zagorevski for his expertise in the proteomics core at Rensselaer Polytechnic Institute and the US National Institutes of Health for support (GM38060).
Author information
Authors and Affiliations
Contributions
M.L. and F.E.L. III contributed experiments and data interpretation. T.N.L. contributed the fractions for analysis and assisted in writing. T.T. contributed the bikunin and assisted in writing. R.J.L. and I.J.A. contributed experimental planning, result interpretation and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results (PDF 4871 kb)
Rights and permissions
About this article
Cite this article
Ly, M., Leach, F., Laremore, T. et al. The proteoglycan bikunin has a defined sequence. Nat Chem Biol 7, 827–833 (2011). https://doi.org/10.1038/nchembio.673
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.673
This article is cited by
-
Simplifying the detection and monitoring of protein glycosylation during in vitro glycoengineering
Scientific Reports (2023)
-
O-Glycoproteomic analysis of engineered heavily glycosylated fusion proteins using nanoHILIC-MS
Analytical and Bioanalytical Chemistry (2022)
-
Glycosaminoglycan Domain Mapping of Cellular Chondroitin/Dermatan Sulfates
Scientific Reports (2020)
-
Shotgun ion mobility mass spectrometry sequencing of heparan sulfate saccharides
Nature Communications (2020)