Abstract
Muscle injury triggers inflammation in which infiltrating mononuclear phagocytes are crucial for tissue regeneration. The interaction of the CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axis that guides phagocyte infiltration is incompletely understood. Here, we show that CX3CR1 deficiency promotes muscle repair and rescues Ccl2−/− mice from impaired muscle regeneration as a result of altered macrophage function, not infiltration. Transcriptomic analysis of muscle mononuclear phagocytes reveals that Apolipoprotein E (ApoE) is upregulated in mice with efficient regeneration. ApoE treatment enhances phagocytosis by mononuclear phagocytes in vitro, and restores phagocytic activity and muscle regeneration in Ccl2−/− mice. Because CX3CR1 deficiency may compensate for defective CCL2-dependant monocyte recruitment by modulating ApoE-dependent macrophage phagocytic activity, targeting CX3CR1 expressed by macrophages might be a powerful therapeutic approach to improve muscle regeneration.
Similar content being viewed by others
Introduction
Monocytes are key players during trauma and inflammation, whereas tissue-resident macrophages have an important role during tissue homeostasis and the resolution of inflammation. However, it is not well established how monocytes and macrophages contribute to both protective/regenerative features and deleterious pathogenic processes. Therefore, defining the molecular and cellular processes that enhance, limit or resolve inflammation may lead to useful therapy. Muscle damage induces massive macrophage infiltration at the injury site, and macrophages are essential for muscle regeneration1,2,3,4. Indeed, partial depletion of mononuclear phagocytes impairs muscle regeneration, whereas reconstitution of myeloid lineage restores regeneration2,5,6. We previously showed that injured skeletal muscle recruits monocytes exhibiting inflammatory profiles that operate phagocytosis and rapidly convert to anti-inflammatory mononuclear phagocytes3. Aside from removing necrotic muscle debris, macrophages promote myogenic cell growth and differentiation by releasing growth factors and cytokines7,8, protect myogenic cells from apoptosis9 and participate in restoring perfusion by promoting collateral artery formation and angiogenesis10. In mice, infiltrating macrophages differentiate from circulating inflammatory Ly6Chigh monocytes that co-express CCR2 and CX3CR1, two important chemokine receptors involved in monocyte motility2,3,11. In mice lacking CCR2 (Ccr2−/−) or its cognate ligand (Ccl2−/−), muscle regeneration and macrophage infiltration are impaired, and macrophage activation is modified5,6,12,13,14,15,16. By contrast, the role of CX3CR1 in muscle healing has not been defined yet, despite its importance in monocyte recruitment17,18, macrophage polarization19,20,21,22, cell survival20,23 and angiogenesis24,25.
In the skeletal muscle, recent works indicated that CX3CR1-negative resident macrophages localized in the normal epimysium/perimysium behave as sensors of pathological events, whereas exudate CX3CR1-expressing mononuclear phagocytes may provide immediate inflammatory response in the injured muscle and longstanding immune surveillance until full muscle restoration26. CX3CR1 is not recognized as a key molecule in resident macrophage ontogeny, although it plays a key role in kidney dendritic cells, brain microglial cells and intestinal macrophages colonization27,28,29. Few studies indicate that the survival of monocytes and tissue macrophages may rely on CX3CR1 as shown within the atherosclerotic plaques30 and in the liver20. More recently, CX3CR1 was identified as a regulator of renal macrophage proliferation31.
Chemokine (CX3C motif) ligand 1 (CX3CL1) also known as fractalkine or neurotactin is the only known member of the CX3C class of chemokines. It is unique because (i) it is expressed both as a membrane-bound and a soluble form and (ii) it binds specifically to its receptor CX3CR1. It is expressed by human and murine primary myoblasts in culture32,33. Whether the CX3CR1/CX3CL1 axis is involved in muscle healing through the recruitment of circulating monocytes or the activation of macrophages, and whether its relation to the CCL2/CCR2 axis is unequivocal, as in atherosclerosis18,34, or ambiguous, as in age-related macular degeneration (AMD)35, remains to be understood.
In this study, we investigate muscle regeneration and mononuclear phagocyte recruitment in a notexin-induced muscle injury model by comparing C57Bl/6 (wild type, WT), Cx3cr1−/−, Ccl2−/− and Cx3cr1−/− Ccl2−/− mice. We show that CX3CR1 deficiency promotes muscle repair and rescues Ccl2−/− mice from impaired muscle regeneration. These phenomena result not from altered mononuclear phagocyte infiltration in Cx3cr1−/− mice but from functional modifications. Transcriptomic analysis were performed to compare muscle mononuclear phagocyte subsets between mice with efficient regeneration (WT, Cx3cr1−/− and Cx3cr1−/− Ccl2−/− mice) and those with poor recovery (Ccl2−/− mice). Among genes differentially expressed, Apolipoprotein E (ApoE) transcripts are downregulated in Ccl2−/− mononuclear phagocyte subsets. Both ApoE administration and ApoE-proficient bone marrow (BM) transfer restore muscle regeneration in Ccl2−/− mice. CX3CR1-ApoE-deficient BM transfer did not rescue CCL2-delayed muscle regeneration as effectively as CX3CR1-deficient ApoE-proficient transfer. Finally, ApoE enhances in vitro phagocytosis by macrophages, restores phagocytic activity in Ccl2−/− mice, and its deficiency leads to incomplete necrotic myofiber removal. Our results suggest that targeting CX3CR1 expressed by mononuclear phagocytes might be useful in alleviating inflammation and promoting muscle regeneration after trauma.
Results
CX3CR1 deficiency promotes muscle healing after injury
We used a mouse model of notexin-induced muscle injury to analyse the muscle reparative process and mononuclear phagocyte recruitment. We show that CX3CR1 (Supplementary Fig. 1a,b) and its ligand CX3CL1 (Supplementary Fig. 1c) are both expressed during muscle regeneration with a maximum reached at day 4. Concomitantly, CCL2 was secreted at days 1 and 4 post injury (Supplementary Fig. 1d). To understand the roles of these chemokines and their functional relationship during muscle repair, we compared muscle regeneration events and mononuclear phagocyte recruitment between C57Bl/6 (WT), Cx3cr1−/−, Ccl2−/− and Cx3cr1−/− Ccl2−/− mice. Notexin induced muscle necrosis (day 1, data not shown), which was followed by leukocyte infiltration (day 4), regenerating myofiber formation (day 10) and myofiber growth (day 21; Fig. 1a). Consistent with published data16, muscle regeneration was impaired in Ccl2−/− mice compared with WT mice and was characterized by delays in necrotic fibre removal and myofiber regeneration (Fig. 1a,b and Supplementary Fig. 2), a smaller cross-sectional area (CSA) of regenerating myofibers (Fig. 1c), more intermuscular adipocytes (Fig. 1d), calcification (Fig. 1e) and fibrosis (Fig. 1f), as well as decreased neovascularization (Fig. 1g). CX3CR1 deficiency improved muscle regeneration: Cx3cr1−/− mice had fewer necrotic and more regenerating myofibers on day 4 (Fig. 1a,b and Supplementary Fig. 2), higher myofiber CSA on days 10 and 21 post injury (Fig. 1c) and reduced intermuscular calcification (Fig. 1e). Strikingly, muscle regeneration in Cx3cr1−/− Ccl2−/− mice was better than in Ccl2−/− mice and similar to that of WT mice for all the indicators studied; it thus enhanced the muscle regeneration improvement driven by the CX3CR1 deficiency (Fig. 1 and Supplementary Fig. 2). These results were unexpected, as CX3CR1 deficiency usually causes severe disorders in various inflammatory models, associated with cell death36, fibrosis development20, delayed wound healing and neovascularization24,25. The observation of the rescued phenotype in both male and female double-deficient mice (Supplementary Fig. 3) but not in Cx3cr1−/− Ccr2−/− compared with Ccr2−/− mice (Supplementary Fig. 4) suggests that a minimum level of monocyte infiltration is required to compensate for the muscle regeneration impairment (monocyte recruitment is more severely impaired in Ccr2−/− than Ccl2−/− mice12). CX3CR1 deficiency thus appears to promote muscle repair and rescue Ccl2−/− mice from impaired muscle regeneration.
Muscle regeneration was assessed after notexin injury in WT, Cx3cr1−/−, Ccl2−/− and Cx3cr1−/− Ccl2−/− mice on days 4, 10 and 21 post injury. (a) Haematoxylin/eosin staining of uninjured and regenerating muscle ( × 100 magnification, scale bar, 100 μm). Arrows indicate dense violet structures corresponding to calcified myofibers, and arrowheads mark intermuscular adipocytes (transparent monovacuolar cells). (b) Quantification of regeneration on day 4 post injury assessed by the percentage of necrotic (infiltrated or not) and regenerating (basophilic+centrally nucleated) myofibers, expressed as a percentage of the total number of myofibers. (c) Quantification of the mean cross-sectional area (CSA) of regenerating myofibers, by image J software. (d) Oil Red O staining of lipids within intermuscular adipocytes, (e) Alizarin Red staining of calcified myofibers, (f) Masson Trichrome staining of connective tissue and quantification of the different areas, by setting appropriate thresholds on image J software. Fat, calcification and fibrosis are expressed as percentages of the total injured area (between 60 and 90% of total muscle surface) and results are a mean of three different muscle cross-sections taken at each end and the middle of the muscle. (d, e and f: scale bar, 100 μm). (g) Vascularization was quantified on muscle cross-sections stained with wheat germ/Griffonia simplicifolia agglutinin (scale bar, 50 μm). Data are mean+s.e.m. of six mice per strain, which represent two distinct experiments. NS, not significant, *P<0.05, **P<0.01 and ***P<0.001 for each knock-out strain versus WT and for Ccl2−/− versus Cx3cr1−/− Ccl2−/− mice.
CCL2 but not CX3CR1 deficiency alters macrophage infiltration
To identify the cellular origin of the muscle regeneration rescue observed in Cx3cr1−/− Ccl2−/− mice, we performed BM transplantation experiments in sub-lethally irradiated Ccl2−/− mice. The control graft produced strongly improved muscle regeneration on day 10 post injury, characterized by increased regenerating myofiber CSA and decreased intermuscular fat accumulation and calcification (Fig. 2a). BM transfer from Cx3cr1−/− mice amplified muscle regeneration rescue and increased the regenerating myofiber CSA. This indicates that the muscle regeneration improvement observed in Cx3cr1−/− mice relied on BM-derived cells.
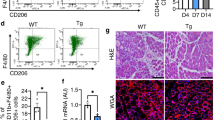
(a) Analysis of muscle regeneration in chimeric Ccl2−/− mice engrafted with bone marrow from Ccl2−/−, WT and Cx3cr1−/− mice, by measuring the mean regenerating myofiber CSA and the areas of intermuscular adipocyte and of calcified myofibers on day 10 post injury. ***P<0.001 for Ccl2−/− mice engrafted with bone marrow from WT or Cx3cr1−/− mice versus Ccl2−/− mice engrafted with Ccl2−/− bone marrow. Data are mean of a single experiment+s.e.m. of three mice per strain. (b) Gating strategy for macrophages on day 4 post injury. Macrophages expressed CD11b, F4/80 and Mgl1 but not Ly6G or NK1.1. Four subsets of cells corresponding to this phenotype were identified; a subset of CD11b+ F4/80+ Ly6Clow Mgl1− cells, with morphology typical of eosinophils, was excluded from the analysis. (c) Proportion of each macrophage subset on day 4 post injury. (d) Kinetics of the four macrophage subsets during muscle regeneration in WT, Cx3cr1−/−, Ccl2−/− and Cx3cr1−/− Ccl2−/− mice. *P<0.05 for Ccl2−/− and Cx3cr1−/− Ccl2−/− strains versus WT. Data are mean of two to four distinct experiments±s.e.m. of at least six mice per strain per time point. (e) The four macrophage subsets from injured muscle were sorted by flow cytometry on day 4 post injury according to the gating strategy described in (b). RNA from one cell-sorting experiment, representing a pool of three mice per strain, was amplified and hybridized on the Illumina whole mouse genome oligo microarrays (WG6). Hierarchical clustered heat map of macrophage subsets 1, 2, 3 and 4 based on the gene expression of ten different clusters. Red: increased expression, green: decreased expression.
As both CX3CR1 and CCL2 are involved in mononuclear phagocyte trafficking, we hypothesized that modulation of mononuclear phagocyte recruitment in the injured muscle might account for the improved regeneration in Cx3cr1−/− mice and rescue of Cx3cr1−/− Ccl2−/− mice. We assessed mononuclear phagocyte infiltration throughout the injured muscle by flow cytometry (Fig. 2b) and identified four distinct populations (subsets 1–4) expressing typical mononuclear phagocyte phenotypic markers (CD11b+ Ly6G− NK1.1− F4/80+ Mgl1+; Fig. 2b and Supplementary Fig. 5). Subset 1 (F4/80low Ly6Chigh) has bean-shaped nuclei and may correspond to infiltrating Ly6Chigh blood monocytes. Subset 2 (F4/80low Ly6Clow) expressed dendritic cell markers, such as CD11c and IAb (major histocompatibility complex molecules-class II), and CD64 (FcγRI) at lower levels than the other three subsets. Cells from subsets 3 and 4 were larger and had typical macrophage morphology; subset 3 (F4/80high Ly6Clow Mgl1low) expressed lower levels of M2-type macrophage markers (such as Mgl1 and CD206) than subset 4 (F4/80high Ly6Clow Mgl1high; Fig. 2b and Supplementary Fig. 5). Cells from subsets 1 and 3 were the main mononuclear phagocyte subsets (about 86%) in muscle at the peak of inflammation, on day 4 post injury (Fig. 2c). In a previous study2, we showed that Ly6C high monocytes in the muscle are recruited from the blood and are non-dividing cells. They switched their phenotype to Ly6Clow mononuclear phagocytes and actively proliferated in situ, contributing to the large amount of mononuclear phagocytes observed in the muscle at day 4 post injury.
Subset 1 was found in significantly lower numbers on day 1 (70% reduction, P<0.0006) as was subset 3 on day 4 (48% reduction, P<0.0001) in injured muscles from Ccl2−/− and Cx3cr1−/− Ccl2−/− compared with WT and Cx3cr1−/− mice (Fig. 2d). This finding confirms that monocyte/macrophage recruitment requires CCL2 (ref. 12). Conversely, CCL2 and CX3CR1 deficiency did not or only slightly affected the proportions of subsets 2 and 4. The greater impairment of the recruitment of subsets 1 and 3 in the injured muscle of Ccr2−/− mice (Supplementary Fig. 6) suggests that their presence in the lesion is necessary for the CX3CR1-dependent compensatory effect. Strikingly, the infiltration kinetics of all mononuclear phagocyte subsets of Ccl2−/− and Cx3cr1−/− Ccl2−/− mice, as for WT and Cx3cr1−/− mice, were superimposable (Fig. 2d) despite strongly different pathophysiological conditions. The apparent lack of association between the muscle regeneration rescue observed in Cx3cr1−/− Ccl2−/− mice and any change in the number of monocytes infiltrating/macrophages proliferating in the injured muscle suggests that the process allowing muscle recovery in Cx3cr1−/− mice may be related to mononuclear phagocyte functions. We next investigated the role of BM-derived macrophages from the various mice strains regarding myogenic precursor cells (MPCs) using in vitro assays. No differences were observed in MPC proliferation or fusion (Supplementary Fig. 7). Neither CX3CR1 nor CCR2 were detected on satellite cells13, on myoblasts or on regenerating myofibers (data not shown).
To address more globally the mononuclear phagocyte functions that may promote muscle regeneration, we performed a genome-wide microarray analysis of the four mononuclear phagocyte subsets from WT, Cx3cr1−/−, Ccl2−/− and Cx3cr1−/− Ccl2−/− muscles on day 4 post injury (GSE73473). One-way analysis of variance analysis identified 434 genes (P<0.05) that distinguished the four subsets (Fig. 2e). Using hierarchical clustering and pathway analysis, we identified 10 clusters that confirmed the original identity and functions of each subset. Because muscle regeneration was not rescued in Cx3cr1−/− Ccr2−/− mice (Supplementary Fig. 4) and muscle infiltration of subsets 1 and 3 was fully abrogated only in Ccr2−/− mice (Supplementary Fig. 5), we postulated that these subsets were mainly responsible for this rescued phenotype and sought to identify in them the genes that promote healing.
ApoE production is adversely regulated by CCL2 and CX3CR1
Comparison of the mononuclear phagocyte transcriptomic profiles from mice with poor regenerative abilities (Ccl2−/−) and from mice with effective muscle healing (WT, Cx3cr1−/− and Cx3cr1−/− Ccl2−/−) identified 22 and 17 genes significantly differentially expressed in mononuclear phagocyte subsets 1 and 3, respectively (Fig. 3a,b). Among them, only ApoE transcripts were downregulated in both Ccl2−/− subsets 1 and 3. We confirmed severely lower ApoE expression in macrophages isolated from injured muscle of Ccl2−/− mice at both RNA (Fig. 3c) and protein levels (Fig. 3d,e and Supplementary Fig. 8). In accordance with the microarray data, ApoE expression was restored in Cx3cr1−/−Ccl2−/− mice. Moreover, we found a higher expression of ApoE in mononuclear phagocytes from CX3CR1-deficient compared with CX3CR1-proficient mice (Fig. 3c–e). We also showed that addition of CX3CL1 on WT macrophages in vitro suppressed ApoE expression by about 30%, without affecting CCL2 expression (Fig. 3f). These results reveal that CX3CL1/CX3CR1 axis is also directly involved in the regulation of ApoE expression.
(a) Venn diagrams illustrating the comparisons between the set of genes significantly differentially regulated in cell subsets 1 and 3 from Ccl2−/− versus WT (in red), Cx3cr1−/− (in green) and Cx3cr1−/− Ccl2−/− (in grey), mice. (b) Heat maps representing the positive (red) and negative (green) log2 ratio of gene expression in subsets 1 (22 genes) and 3 (17 genes) from Ccl2−/− mice compared with WT, Cx3cr1−/− and Cx3cr1−/− Ccl2−/− mice. ApoE expression in the global pool of mononuclear phagocytes extracted from injured muscle though magnetic columns (CD11b+ Ly6G− NK1.1−) was analysed for each mouse strain on day 4 post injury by: (c) Real time-PCR (data are a mean+s.e.m. of three experiments). (d) Western blotting (one experiment representative of two; first lane: size marker in kDa), (e) immunolabelling and quantification of mean fluorescent intensity (MFI), with the appropriate threshold using image J software. (Data are a mean+s.e.m. of at least five fields counted (magnification of × 40) from two cell sorting experiments.) NS, not significant, *P<0.05, **P<0.01 and ***P<0.001 for each knock-out strain versus wild type. (f) Modifications in ApoE and Ccl2 RNA expression induced after CX3CL1 (100 nM) in vitro stimulation during 16 h was analysed by real-time PCR in the global pool of mononuclear phagocytes extracted from WT mice, compared with Cx3cr1−/−. Data are a mean+s.e.m. for six mice per strain in three distinct experiments; NS, not significant, **P<0.01. (g) Network diagram showing the links between most of the genes differentially up- (red) or downregulated (green) in Ccl2−/− mononuclear phagocytes compared with Cx3cr1−/− Ccl2−/− mononuclear phagocytes, inflammatory response, endocytosis signalling, LXR/RXR activation and lipid metabolism (Ingenuity Pathway analysis).
Interestingly, most of the dysregulated genes in mononuclear phagocytes from Ccl2−/− versus WT, Cx3cr1−/− and Cx3cr1−/− Ccl2−/− mice can be linked in a network in which ApoE is central and related to inflammatory response, endocytosis signalling and the LXR/RXR activation pathway involved in the regulation of inflammation and lipid metabolism37 (Fig. 3g). We previously published a functional link between ApoE and CX3CR1 in AMD38. Sub-retinal macrophages in AMD patients as well as in sub-retinal inflammation observed in Cx3cr1−/− mice express high levels of ApoE. Previous studies indicate that ApoE is an immunomodulatory agent that polarizes macrophages in a M2 anti-inflammatory phenotype39. We previously showed that M2 macrophages are associated with myofiber growth and myoblast differentiation and fusion3. In addition, ApoE can suppress the pro-inflammatory response mediated by microbial stimuli in vivo40,41. Furthermore, the delay in skeletal muscle healing in ApoE−/− mice following hindlimb ischaemia-reperfusion42 suggests that this gene plays a major role in the muscle regeneration process.
ApoE favours phagocytosis and muscle regeneration
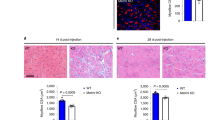
Histological analysis of injured muscle from ApoE−/− mice 10 days after injury revealed strongly impaired muscle regeneration, with a markedly lower CSA of regenerating myofiber and increased intermuscular fat and calcification (Fig. 4a). These results are consistent with previous reports that ApoE−/− mice develop calcification and accumulate lipid-filled foam cells within atheromatous plaque when fed a high-fat diet43,44. Using hydrodynamic-based in vivo transfection, we next showed that systemic expression of human ApoE3 improved muscle repair in Ccl2−/− mice (Fig. 4b). In addition, Cx3cr1−/− ApoE−/− bone-marrow transfer in irradiated Ccl2−/− mice did not promote myofiber growth as effectively as Cx3cr1−/− bone-marrow transfer (+33% myofiber CSA versus +58%; Fig. 4c). We also observed high reduction of myofiber growth in Cx3cr1−/− Ccl2−/− ApoE−/− mice compared with Cx3cr1−/− Ccl2−/− mice (Fig. 4d). These results demonstrate that the impairment of muscle regeneration in Ccl2−/− mice depends at least partially on the inability of muscle mononuclear phagocytes to produce enough ApoE and that CX3CR1 deficiency promotes and rescues Ccl2−/− muscle healing by regulating ApoE production by macrophages.
(a) Analysis of muscle regeneration of ApoE−/− (n=11) versus WT mice (n=7) on day 10 post injury, by measurement of mean CSA of regenerating myofibers, myofiber density and areas of fat and calcification. Data are mean+s.e.m. of two distinct experiments. (b) Analysis on day 10 post injury of muscle regeneration in Ccl2−/− mice, transfected (n=3) or not (n=3) with a pLIVE plasmid encoding for human ApoE3, by measurement of mean CSA of regenerating myofibers, myofiber density and areas of fat and calcification. In this experimental system, systemic expression of ApoE3 was detected for at least 8 days after transfection (data not shown). Data are mean+s.e.m. of a single experiment. Scale bar, 100 μm. (c) Analysis of muscle regeneration in chimeric Ccl2−/− mice engrafted with Ccl2−/− (n=5), Cx3cr1−/− (n=5) or Cx3cr1−/− ApoE−/− (n=4) bone marrow, by measurement of the mean CSA of regenerating myofibers on day 21 post injury. (d) Analysis of muscle regeneration in Cx3cr1−/− Ccl2−/− mice (n=4) and Cx3cr1−/− Ccl2−/− ApoE−/− mice (n=4). Data are mean+s.e.m.; NS, not significant, *P<0.05, **P<0.01 and ***P<0.001.
Because macrophage phagocytic activity is impaired in ApoE−/− (Supplementary Fig. 9) and improved in Cx3cr1−/− mice21,45, we hypothesized that improved muscle regeneration in Cx3cr1−/− mice and rescue in Cx3cr1−/− Ccl2−/− mice might be linked to increased phagocytosis, and indeed we measured more phagocytic activity in muscles from Cx3cr1−/− compared with WT mice (Fig. 5a). The better necrotic myofiber removal in Cx3cr1−/− Ccl2−/− compared with Ccl2−/− mice (for an equivalent number of infiltrated mononuclear phagocytes) suggests that CX3CR1-deficient mononuclear phagocytes have higher phagocytic activity. Ex vivo experiments confirmed that muscle macrophages from Cx3cr1−/− and Cx3cr1−/− Ccl2−/− mice engulfed fluorescent microspheres more effectively than those from Ccl2−/− animals (Fig. 5b). Moreover, addition of exogenous APOE3 improved both phagocytic activity of Ccl2−/− muscle macrophages in vitro (Fig. 5c) and necrotic myofibre removal in Ccl2−/− mice (Fig. 5d). Addition of CCL2 restored phagocytic activity of Ccl2−/− macrophages (Fig. 5e, right panel) but not that of WT and Cx3cr1−/− macrophages. A reduction in WT macrophage phagocytic capacity was observed after incubation with CX3CL1 (Fig. 5e, left panel). Finally, the addition of CX3CL1 and CCL2 on WT macrophage-enriched muscular cell suspensions triggered similar expression of molecules involved in inflammatory response such as IL-1β, IL-1RA (IL-1 receptor antagonist) and iNOS (inducible nitric oxide synthase; Fig. 5f). Altogether, our results suggest that impaired muscle healing in Ccl2−/− mice is linked to decreases in monocyte/macrophage recruitment and function, notably phagocytosis.
(a) Quantification of in vivo phagocytosis, by assessment of the numbers of infiltrated necrotic myofibers versus total necrotic myofibers infiltrated or not on day 4 post injury. Scale bar, 100 μm. Data are means+s.e.m. of six mice per strain in two independent experiments. (b) Phagocytosis activity of mononuclear phagocytes extracted from injured-muscle of WT, Cx3cr1−/−, Ccl2−/− and Cx3cr1−/− Ccl2−/− mice on day 4 post injury was assessed by incubating muscle cells for 4 h with red fluorescent microspheres. This parameter is expressed as the number of cells that engulfed at least one bead per 10,000 cells. Results are means+s.e.m. of six mice in three independent experiments. (c) Phagocytosis activity of Ccl2−/− muscle mononuclear phagocytes in the absence or presence of exogenous human APOE3 protein. Results are mean±s.e.m. of five mice in two independent experiments. (d) Quantification on day 4 post injury of Ccl2−/− myofiber removal in the absence (n=2) or presence (n=4) of exogenous human APOE3 protein. (e) Phagocytosis activity of mononuclear phagocytes from injured-muscle of WT, Cx3cr1−/− and Ccl2−/− mice on day 4 post injury was assessed 16 h after incubation of muscle cells with CX3CL1 or CCL2 chemokines. The numbers of muscle cells that engulfed at least one fluorescent microsphere were compared between treated and untreated cells for all mice strains. (f) Effect of CX3CL1 and CCL2 on IL-1, IL-1 receptor antagonist (Ra) and iNOS (nitric oxide synthase) expression was measured by real-time PCR in mononuclear phagocytes extracted from WT mice incubated during 16 h with CX3CL1 (100 nM) or CCL2 (100 nM), and compared with untreated cells. Data are mean+s.e.m. of six mice per strain in three independent experiments. NS, not significant, *P<0.05, **P<0.01 and ***P<0.001.
Discussion
Skeletal muscle regeneration is a complex series of events, in which monocytes/macrophages play a major part in the healing process. Chemokines and their receptors orchestrate their distribution and their functions to ensure muscle regeneration. CX3CR1 and CCL2 deficiencies affect post-lesion blood monocytosis differently: CX3CR1 deficiency is associated with a reduction in Ly6Clow monocytes, whereas CCL2 deficiency affects the number of Ly6Clow and Ly6Chigh monocytes (Supplementary Fig. 10). Because Ly6Clow blood monocytes have no role in the muscle regeneration process3,46, their lack may not impair this process. Few groups including Saederup et al.47 and our group18 have shown additive or synergistic effects of CCR2 and CX3CR1 axis on monocyte recruitment or macrophage accumulation. CCR2 and CX3CR1 axis may also lead to the opposite effect, and more specifically CX3CR1 deficiency may promote CCR2-dependent microglial cell accumulation in the retina35. Here we could not detect any changes in monocyte recruitment or in macrophage accumulation consistently with our previous work showing that monocyte recruitment in the injured muscle was exclusively dependent on CCR2 axis3. In these conditions, CX3CR1 may only affect macrophage functions, not monocyte recruitment or macrophage proliferation.
CX3CR1 is an essential pleiotropic chemokine receptor involved in various biological functions, including cell chemotaxis, apoptosis, cell activation/polarization, organ development and neovascularization. Its deficiency is considered deleterious in a large variety of acute inflammatory responses where it exacerbates inflammation20,24,36. In chronic diseases, such as atherosclerosis, CX3CR1 deficiency also limits monocyte recruitment and macrophage accumulation at inflammatory sites leading to reduced atherosclerosis18,48,49. In contrast, CX3CR1 presence is somewhat protective in AMD50. The role of CX3CR1 appears to be different depending on the inflammatory model. Our results show for the first time that CX3CR1 deficiency can improve wound healing in an acute injury model without affecting monocyte recruitment or macrophage accumulation. In the mdx mice, a chronic model of muscle injury (a murine model for Duchenne muscular dystrophy), previous studies using gene expression microarrays have demonstrated that dystrophic muscles are characterized by an inflammatory ‘molecular signature’51,52, in which CC chemokines are prominent and among which CCL2 and its receptor CCR2 are highly expressed53,54. There is no evidence of CX3CR1/CX3CL1 expression in this context and the role of CX3CR1 has not yet been investigated. In a previous work9, we showed that four molecular systems including CX3CR1/CX3CL1 are involved in MPC apoptosis inhibition driven by macrophages in vivo and in vitro. Although apoptosis induction is fairly easy to achieve in vitro (through the incubation with various pro-apoptotic drugs), the MPC apoptosis events in vivo are rather rare, with apoptotic MPC representing only a very small number of cells in the myoblast population. In contrast, phagocytosis events during muscle repair are ubiquitous, and it is more likely that macrophages will engulf apoptotic MPC in vivo than protect them through the CX3CR1/CX3CL1 molecular system.
Figure 6 summarizes the main findings of the work and proposes a model of action of macrophages in muscle regeneration via ApoE production. We demonstrated that CX3CR1 deficiency is associated with increased removal of necrotic myofibers, as it is with the phagocytosis of amyloid-beta deposits in the brain of a murine model of Alzheimer’s disease21. Similarly, CX3CR1-deficient mononuclear phagocytes overexpressed ApoE protein, which is known to stimulate macrophage phagocytosis and dampen inflammatory response39,45. Moreover, a recent study38 demonstrated a link between ApoE and CX3CR1 in AMD. Sub-retinal macrophages in AMD patients as well as in sub-retinal inflammation observed in Cx3cr1−/− mice express high levels of ApoE. In addition, ApoE deletion in Cx3cr1−/− mice prevents pathogenic- and stress-induced subretinal macrophage accumulation. Here we showed that the exogenous administration of ApoE enhanced CCL2-deficient mononuclear phagocyte phagocytic activity. These results suggest that defective CCL2-dependent monocyte recruitment may be compensated by CX3CR1 deficiency through the modulation of ApoE-dependent macrophage phagocytic activity. Therefore, therapeutic intervention to improve mononuclear phagocyte functions by targeting the CX3CR1 chemokine axis might control inflammation and enhance muscle regeneration in response to injury.
Methods
Mice
Experiments were conducted on adult female and male mice (8–16 weeks old) of the following strains (all on a C57Bl/6J genetic background): C57Bl/6J (WT; purchased from Janvier), Cx3cr1−/− (ref. 55), Ccl2−/− (#004434, JAX), Ccr2−/− (#004999, JAX), ApoE−/− (#002052, JAX), Cx3cr1gfp/+ (#008451, JAX) were intercrossed to obtain Cx3cr1−/− Ccl2−/−, Cx3cr1−/− ApoE−/−, Cx3cr1−/− Ccl2−/− ApoE−/− and CX3CR1gfp/gfp ApoE−/− as described in ref. 18. Mice were bred and maintained in our specific pathogen-free animal facility according to institutional guidelines and used according to the French legislation (no. A-75-1315). All experiment protocols were approved by the local ethics committee and validated by the Service Protection et Santé Animales, Environnement (no. A-75-2065).
Muscle injury
Muscle injury was caused by intramuscular injection of 10 μl of notexin (25 μg ml−1 in PBS; Latoxan) into the tibialis anterior (TA) muscle. Muscle and fascia were harvested together for analysis at different time points post injury (1, 4, 7, 10, 14, 21 days). In some experiments, a pLIVE plasmid encoding for human ApoE3 was injected into mouse tail veins 6 h before muscle injury.
Histological analysis
TA muscles were removed, snap frozen in nitrogen-chilled isopentane and kept at −80 °C until use for histological analysis. Cryosections of 7 μm thick were prepared for various types of histological staining. Muscle regeneration was analysed quantitatively for the entire injured area (between 60 and 90% of the entire muscle). The percentages of necrotic and regenerating myofibers as well as the CSA of the regenerating myofibers, an established indicator, were quantified on haematoxylin/eosin-stained muscle sections recorded with an Olympus BX51 at × 10 magnification, connected to an evolution VF camera (medicaCybernetics). We used image J software to count three fields always chosen in the same overall injured area (representing 660±150 myofibers) for at least six different mice analysed per time point. Regenerating myofiber density was assessed in the same fields and is expressed as the number of regenerating myofibers per field. Phagocytosis was assessed on day 4 post injury on muscle cross-sections stained with haematoxylin/eosin by counting the ratio of infiltrated to non-infiltrated necrotic myofibers. Vascularization was quantified on muscle cross-sections stained with wheat germ/Griffonia simplicifolia agglutinin. We counted 8±1 fields (at × 20 magnification) chosen randomly over the entire injured area and representing 485±160 myofibers, in three different mice. Areas of fat, calcification and fibrosis were evaluated over the entire injured area of three muscle cross-sections at levels spaced 900 μm apart, stained, respectively, with Oil Red O, Alizarin Red and Masson Trichrome and recorded with a Nikon AZ100 macroscope connected to a DS-Ri1 camera (Nikon). Appropriate thresholds were set with image J software to evaluate these areas. Each parameter was studied on days 4 (except fibrosis), 10 and 21 post injury in at least six different mice.
Isolation of leukocytes and macrophages from muscle
Mice were flushed with 10 ml of PBS to remove blood. Muscles and fascia were weighed together, minced and digested twice for 45 min each time in DMEM containing collagenase B 0.2% (Roche Diagnostics) and trypsin-EDTA 0.2% at 37 °C. The resulting homogenate was filtered through a 70-μm cell strainer. In some experiments, macrophages were isolated from muscle cell suspension using MACS technology (Miltenyi Biotec). A negative selection using PE-Ly6G and PE-NK1.1 antibodies and anti-PE magnetic beads (to remove neutrophils and NK cells) was followed by a positive selection of macrophages with magnetic beads coupled with CD11b antibodies.
Flow cytometry analysis and cell sorting
Cell suspensions were incubated with anti-mouse FcγII/III receptor (clone 2.4G2, BD Biosciences, 1:100) for 10 min at 4 °C in FACS buffer (Ca2+/Mg2+-free PBS with 0.5% BSA) and then stained with the following fluorescent-conjugated anti-mouse antibodies: V450-Ly6G (clone 1A8, BD Biosciences, 1:100), FITC-CD11c (clone HL3, BD Biosciences, 1:100), PE-Cy7-NK1.1 (clone PK136, BD Biosciences, 1:100), APC-Cy7-Ly6C (clone AL-21, BD Biosciences, 1:100), PE-CD64 (clone X54-5/7.1, BD Biosciences, 1:100), APC-IAb (major histocompatibility complex molecules-class II, clone AF6-120-1, BD Biosciences, 1:200), PE-SiglecF (clone E50-2440, BD Biosciences, 1:100), PE-CD206 (clone MR5D3, BD Biosciences, 1:100), biotinylated anti-Mgl1 (MCA2392B, Serotec, 1:100), PE-CCR2 (clone 475301, R&D Systems, 1:100), PerCP-Cy5.5-CD11b (clone M1/70, ebiosciences, 1:500) and APC-F4/80 (clone BM8, ebiosciences, 1:150). A PE-streptavidin (1:1,500, BD Biosciences) was used to reveal Mgl1 expression. Cells were analysed on a FACS Canto II (BD Biosciences) with Diva and FlowJo softwares. Sorting of macrophage cell subsets was performed on a FACSAria II cell sorter (BD Biosciences).
Microarray analysis, normalization, data set analysis
The four subsets of mononuclear phagocytes were sorted from the injured muscle by flow cytometry on day 4 post injury with the gating strategy showed in Fig. 2. RNA was amplified with the Ovation Pico WTA system V2 (NuGEN) and the resulting complementary DNA (cDNA) was hybridized on Illumina whole mouse genome oligo microarrays (WG6 V2.0). Array data were analysed with Genome studio (Illumina) and MeV software. Filter on background signals and quantile normalization were applied, and only probe sets showing significance (P-value<0.05) in 8 of the 16 samples were selected. One-way analysis of variance (P<0.01) with subsequent hierarchical clustering was performed on median centred log2 intensity of the resulting 2,472 probes and identified 434 genes that discriminated between the four cell subsets and spread into 10 clusters. Genes showing differential expression in Ccl2−/− mice compared with WT, Cx3cr1−/− and Cx3cr1−/− Cc2−/− were selected according to Eisen et al.56 and comparisons are presented with Venn diagrams (http://bioinfogp.cnb.csic.es/tools/venny/index.html). Ingenuity Pathway Analysis (IPA; Ingenuity Systems Inc.) software was used to identify metabolic and signalling pathways.
Quantitative RT–PCR
Total RNA from cell lysates of injured muscles was extracted using the RNeasy Mini Kit (Qiagen), according to the manufacturer’s instructions. Reverse transcription was performed on 10 ng of RNA using SuperScript VILO cDNA Synthesis Kit (Invitrogen). cDNA were quantified by qPCR using Power SYBR Green PCR Master Mix (Life Technologies) and ApoE, Cx3cr1, Ccl2, Il-1β, Il-1Ra (receptor antagonist), iNOS primers were added at 250 nM each in a final volume of 20 μl (in duplicate for each sample). The sequences of primers used are as follows: ApoE-F: 5′- CCTGAACCGCTTCTGGGATT -3′, ApoE-R:5′- CGTAGATCCTCCATGTCGGC -3′, Cx3cr1-F: 5′- TTCAATCCCAAGGCCCTGTC -3′, Cx3cr1-R: 5′- GTGCAAGCAACAGAGTTGGG -3′, Il1β-F: 5′- CTGTGTCTTTCCCGTGGACC -3′, Il1β-R: 5′- CAGCTCATATGGGTCCGACA -3′, Il1ra-F:5′- TCACCCATGGCTTCAGAGGCAGCC -3′, Il1ra-R: 5′- GGCCTTTCTCAGAGCGGATGAAG -3′, Inos-F: 5′- CCAAGCCCTCACCTACTTCC -3′, Inos-R: 5′- CTCTGAGGGCTGACACAAGG -3′. PCR (denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C (15 s) and 60 °C (60 s)) were performed on an ABI7300 (Applied Biosystems). Relative target gene quantification was normalized to GAPDH as housekeeping gene control for each sample and to untreated sample for each experiment, using the 2 –ΔΔCt method.
ELISA assays
Muscles obtained at day 0, 1, 4, 10 and 21 after notexin-injury were stored at −20 °C, thawed and centrifuged to obtain tissue homogenates (1 muscle in 1 ml PBS). Murine ELISA CX3CL1 (Fractalkine) and CCL2 (MCP1) assays were performed using Quantikine ELISA Mouse Immunoassay kits (Bio-techne) following the manufacturer’s protocol. ApoE3 was measured in mouse serum on day 8 post injury with a HRP-human ApoE3 ELISA Kit (Mabtech).
Western blot
Macrophages sorted from injured muscle on day 4 post injury through MACS technology were pelleted and lysed in PBS pH7.4, 2% Triton X-100, 1% SDS complemented with antiprotease cocktail (Roche). The samples were then assayed for protein content, diluted in sample loading buffer and heated for 5 min at 95 °C. Proteins (45 μg per lane) were separated by 4-20% standard SDS–polyacrylamide gel electrophoresis. Blots were probed with polyclonal antibodies against ApoE (Abcam, ab20874, 1:750) and tubulin (Millipore, CP06, 50 ng ml−1).
Immunolabelling
Frozen muscle cryosections were stained using rabbit anti mouse-MyoD (M-318, SantaCruz) and monoclonal mouse anti-myogenin (F5D, DSHB) antibodies (green) were visualized with Alexa Fluor-594- and -488-conjugated secondary antibodies (Invitrogen) before mounting in Vectashield mounting medium containing 4,6-diamidino-2-phenylindole (Vector Laboratories). Macrophages sorted from injured muscle on day 4 post injury through MACS technology were spun onto glass slides and kept at −80 °C until use. Apolipoprotein E immunolabelling used a rabbit polyclonal anti-mouse ApoE (Abcam, 1/30e), and an anti-rabbit Alexa 488 antibody (Life Technology) as a secondary antibody.
BM transplantation
BM transplantation was performed as follows: donor BM cells were obtained by flushing femoral and tibial BM of WT, Ccl2−/−, Cx3cr1−/−, Cx3cr1GFP/GFP or Cx3cr1GFP/GFP ApoE−/− mice with PBS. Cells were filtered, washed twice and counted in a Malassez slide. 10 × 106 BM cells in 0.1 ml of PBS was injected retro-orbitally to 10.0-Gy-irradiated Ccl2−/− mice (5 Gy twice, 3 h apart) directly, at the end of the second irradiation. Chimerism was evaluated on mice engrafted with CX3CR1gfp/gfp or CX3CR1gfp/gfpApoE−/− BM, calculating the percentage of GFP+ cells among the total number of CD11b+ CD115+ F4/80+ monocytes. Muscle injury was performed 10 weeks after BM transplantation.
Macrophage and MPC culture
Macrophages were obtained from BM precursor cells. In brief, total BM was obtained from mice by flushing femur and tibia BM with DMEM. Cells were cultured in a DMEM-containing conditioned medium of L929 cell line (enriched in M-CSF) for 7 days. After washing, DMEM-serum-free medium was added for 24 h, and cells were recovered and centrifuged in order to obtain macrophage-conditioned medium. For phagocytosis studies, cell cultures were performed under standard conditions in DMEM/F12 (Gibco) containing 20% FBS and 2% Ultroser G serum (Pall) during 16 h with or without addition of recombinant recombinant human cytokines CX3CL1 (R&D Systems) or CCL2 (PeproTech) at 100 nM.
Murine MPCs were obtained from TA muscle and cultured with standard conditions in DMEM/F12 (Gibco) containing 20% FBS and 2% Ultroser G serum (Pall). For proliferation studies, MPCs were seeded at 10,000 cells per cm2 on Matrigel (1/10) and incubated for 1 day with macrophage-conditioned medium+2.5% FBS. Then, cells were incubated with anti-ki67 antibodies (15580, Abcam). For fusion index studies, MPCs were seeded at 30,000 cells per cm2 on Matrigel (1/10) and incubated for 3 days with macrophage-conditioned medium containing 2% horse serum. Then, cells were incubated with antibodies against desmin (32362, Abcam).
Phagocytosis
Macrophage-enriched muscular cell suspensions extracted at day 4 post injury were cultured for 4 h with red fluorescent (580/605) Fluospheres carboxylate-modified microspheres (0.2 μm size) at a 1:10 ratio (macrophage/microspheres) in six-well plates. Cells were analysed on a Zeiss inverted fluorescent axio observer Z1 microscope. Phagocytosis was expressed as the number of macrophages containing at least one microsphere per 1,000 cells, counted using and Image J software, in three distinct experiments. For some experiments, macrophage-enriched muscular cell suspensions were cultured for 16 h with or without addition of Apolipoprotein E3 (Leinco Technologies) at 10 μg ml−1, recombinant human cytokines CX3CL1 (R&D Systems) or CCL2 (PeproTech) at 100 nM.
In vivo phagocytosis was assessed on day 4 post injury as the percentage of phagocytosed myofibers (infiltrated by mononuclear cells) among the total number of necrotic (not infiltrated)+phagocytosed myofibers. In some case, Apolipoprotein E3 was injected in the muscle, twice per day (2 × 10 μg), for 3 days.
Statistical analyses
Data are presented as the mean plus the standard error of the mean (s.e.m.). Statistical analyses compared the groups with a Mann–Whitney test or with a Wilcoxon test for matched paired values (Prism software). P values of less than 0.05 were considered statistically significant.
Additional information
Accession number: Microarray data have been deposited in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/gds) under accession code GSE73473.
How to cite this article: Arnold, L. et al. CX3CR1 deficiency promotes muscle repair and regeneration by enhancing macrophage ApoE production. Nat. Commun. 6:8972 doi: 10.1038/ncomms9972 (2015).
Accession codes
References
McLennan, I. S. Degenerating and regenerating skeletal muscles contain several subpopulations of macrophages with distinct spatial and temporal distributions. J. Anat. 188, 17–28 (1996).
Summan, M. et al. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1488–R1495 (2006).
Arnold, L. et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 (2007).
Mounier, R. et al. AMPKalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 18, 251–264 (2013).
Shireman, P. K. et al. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J. Leukoc. Biol. 81, 775–785 (2007).
Contreras-Shannon, V. et al. Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2-/- mice following ischemic injury. Am. J. Physiol. Cell. Physiol. 292, C953–C967 (2007).
Cantini, M. & Carraro, U. Macrophage-released factor stimulates selectively myogenic cells in primary muscle culture. J. Neuropathol. Exp. Neurol. 54, 121–128 (1995).
Saclier, M., Cuvellier, S., Magnan, M., Mounier, R. & Chazaud, B. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 280, 4118–4130 (2013).
Sonnet, C. et al. Human macrophages rescue myoblasts and myotubes from apoptosis through a set of adhesion molecular systems. J. Cell Sci. 119, 2497–2507 (2006).
Cochain, C. et al. Regulation of monocyte subset systemic levels by distinct chemokine receptors controls post-ischaemic neovascularization. Cardiovasc. Res. 88, 186–195 (2010).
Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Lu, H., Huang, D., Ransohoff, R. M. & Zhou, L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J. 25, 3344–3355 (2011).
Lu, H. et al. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 25, 358–369 (2011).
Martinez, C. O. et al. Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R832–R842 (2010).
Ochoa, O. et al. Delayed angiogenesis and VEGF production in CCR2-/- mice during impaired skeletal muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R651–R661 (2007).
Shireman, P. K., Contreras-Shannon, V., Reyes-Reyna, S. M., Robinson, S. C. & McManus, L. M. MCP-1 parallels inflammatory and regenerative responses in ischemic muscle. J. Surg. Res. 134, 145–157 (2006).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Combadiere, C. et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117, 1649–1657 (2008).
Aoyama, T., Inokuchi, S., Brenner, D. A. & Seki, E. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology 52, 1390–1400 (2010).
Karlmark, K. R. et al. The fractalkine receptor CX(3)CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 52, 1769–1782 (2010).
Liu, Z., Condello, C., Schain, A., Harb, R. & Grutzendler, J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-beta phagocytosis. J. Neurosci. 30, 17091–17101 (2010).
Mizutani, N. et al. Dose-dependent differential regulation of cytokine secretion from macrophages by fractalkine. J. Immunol. 179, 7478–7487 (2007).
Blomster, L. V. et al. CX(3)CR1 deficiency exacerbates neuronal loss and impairs early regenerative responses in the target-ablated olfactory epithelium. Mol. Cell. Neurosci. 48, 236–245 (2011).
Clover, A. J., Kumar, A. H. & Caplice, N. M. Deficiency of CX3CR1 delays burn wound healing and is associated with reduced myeloid cell recruitment and decreased sub-dermal angiogenesis. Burns 37, 1386–1393 (2011).
Ishida, Y., Gao, J. L. & Murphy, P. M. Chemokine receptor CX3CR1 mediates skin wound healing by promoting macrophage and fibroblast accumulation and function. J. Immunol. 180, 569–579 (2008).
Brigitte, M. et al. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheum. 62, 268–279 (2010).
Hochheiser, K. et al. Exclusive CX3CR1 dependence of kidney DCs impacts glomerulonephritis progression. J. Clin. Invest. 123, 4242–4254 (2013).
Mizutani, M. et al. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J. Immunol. 188, 29–36 (2012).
Medina-Contreras, O. et al. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J. Clin. Invest. 121, 4787–4795 (2011).
Landsman, L. et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 113, 963–972 (2009).
Engel, D. R. et al. CX3CR1 reduces kidney fibrosis by inhibiting local proliferation of profibrotic macrophages. J. Immunol. 194, 1628–1638 (2015).
Chazaud, B. et al. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J. Cell Biol. 163, 1133–1143 (2003).
Griffin, C. A., Apponi, L. H., Long, K. K. & Pavlath, G. K. Chemokine expression and control of muscle cell migration during myogenesis. J. Cell Sci. 123, 3052–3060 (2010).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007).
Sennlaub, F. et al. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol. Med. 5, 1775–1793 (2013).
Cardona, A. E. et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9, 917–9245 (2006).
Freeman, M. W. & Moore, K. J. eLiXiRs for restraining inflammation. Nature Med. 9, 168–169 (2003).
Levy, O. et al. Apolipoprotein E promotes subretinal mononuclear phagocyte survival and chronic inflammation in age-related macular degeneration. EMBO Mol. Med. 7, 211–226 (2015).
Baitsch, D. et al. Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler. Thromb. Vasc. Biol. 31, 1160–1168 (2011).
Van Oosten, M. et al. Apolipoprotein E protects against bacterial lipopolysaccharide-induced lethality. A new therapeutic approach to treat gram-negative sepsis. J. Biol. Chem. 276, 8820–8824 (2001).
Ali, K., Middleton, M., Pure, E. & Rader, D. J. Apolipoprotein E suppresses the type I inflammatory response in vivo. Circ. Res. 97, 922–927 (2005).
Kang, J. et al. Apolipoprotein E-/- mice have delayed skeletal muscle healing after hind limb ischemia-reperfusion. J. Vasc. Surg. 48, 701–708 (2008).
Naik, V. et al. Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study. Cardiovasc. Res. 94, 545–554 (2012).
Li, A. C. & Glass, C. K. The macrophage foam cell as a target for therapeutic intervention. Nat. Med. 8, 1235–1242 (2002).
Grainger, D. J., Reckless, J. & McKilligin, E. Apolipoprotein E modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein E-deficient mice. J. Immunol. 173, 6366–6375 (2004).
Varga, T. et al. Tissue LyC6- macrophages are generated in the absence of circulating LyC6- monocytes and Nur77 in a model of muscle regeneration. J. Immunol. 191, 5695–5701 (2013).
Saederup, N., Chan, L., Lira, S. A. & Charo, I. F. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation 117, 1642–1648 (2008).
Fumagalli, S., Perego, C., Ortolano, F. & De Simoni, M. G. CX3CR1 deficiency induces an early protective inflammatory environment in ischemic mice. Glia 61, 827–842 (2013).
Donnelly, D. J. et al. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J. Neurosci. 31, 9910–9922 (2011).
Combadiere, C. et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Invest. 117, 2920–2928 (2007).
Chen, Y. W., Zhao, P., Borup, R. & Hoffman, E. P. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J. Cell Biol. 151, 1321–1336 (2000).
Porter, J. D. et al. A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum. Mol. Genet. 11, 263–272 (2002).
Fang, J., Shi, G. P. & Vaghy, P. L. Identification of the increased expression of monocyte chemoattractant protein-1, cathepsin S, UPIX-1, and other genes in dystrophin-deficient mouse muscles by suppression subtractive hybridization. J. Cell. Biochem. 79, 164–172 (2000).
Porter, J. D. et al. Persistent over-expression of specific CC class chemokines correlates with macrophage and T-cell recruitment in mdx skeletal muscle. Neuromuscul. Disord. 13, 223–235 (2003).
Combadiere, C. et al. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation 107, 1009–1016 (2003).
Eisen, M. B., Spellman, P. T., Brown, P. O. & Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl Acad. Sci. USA 95, 14863–14868 (1998).
Acknowledgements
This work was supported by the European Community’s Seventh Framework Project FP7-Health-2009 ENDOSTEM 241440, Association Française contre les Myopathies (AFM) 15775, the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Université Pierre et Marie Curie (UPMC). We are deeply grateful to Olivier Levy and Florian Sennlaub from the ‘Institut national de la vision’ for the generous gift of ApoE3-pLIVE and pLIVE plasmid controls.
Author information
Authors and Affiliations
Contributions
L.A. and H.P. designed and performed the experiments, analysed the data and wrote the manuscript. C.B.deC., M.S., P.H., L.P., E.G., F.L., J.V., R.M. and W.C. performed the experiments and analysed the data. B.Ch. and A.B. gave technical support and conceptual advice. N.B. conducted all bioinformatics analyses. B.C. and C.C. designed experiments, wrote the manuscript and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-10. (PDF 1618 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Arnold, L., Perrin, H., de Chanville, C. et al. CX3CR1 deficiency promotes muscle repair and regeneration by enhancing macrophage ApoE production. Nat Commun 6, 8972 (2015). https://doi.org/10.1038/ncomms9972
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms9972
This article is cited by
-
A novel interaction between CX3CR1 and CCR2 signalling in monocytes constitutes an underlying mechanism for persistent vincristine-induced pain
Journal of Neuroinflammation (2018)
-
Crossroads between peripheral atherosclerosis, western-type diet and skeletal muscle pathophysiology: emphasis on apolipoprotein E deficiency and peripheral arterial disease
Journal of Biomedical Science (2017)
-
Analysis of monocyte infiltration in MPTP mice reveals that microglial CX3CR1 protects against neurotoxic over-induction of monocyte-attracting CCL2 by astrocytes
Journal of Neuroinflammation (2017)
-
Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates
Nature Materials (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.