Abstract
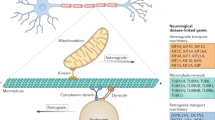
Hereditary axonal peripheral neuropathies comprise a genetically heterogeneous group of disorders that are clinically subsumed under the name of Charcot–Marie–Tooth (CMT) disease type 2 (CMT2). Historically, two classes of CMT have been differentiated: demyelinating forms of CMT (CMT1), in which nerve conduction velocities are decreased, and the axonal CMT2 forms, in which nerve conduction velocities are preserved. Recently, a number of genes that are defective in patients with the main forms of CMT2 have been identified. The molecular dissection of cellular functions of the related gene products has only just begun, and detailed pathophysiological models are still lacking. The known CMT2-related genes represent key players in these pathways, however, and are likely to provide powerful tools for identifying targets for future therapeutic intervention.
Key Points
-
Traditionally, the Charcot–Marie–Tooth (CMT) neuropathies fall into two main groups: demyelinating forms (CMT1), in which nerve conduction velocities are reduced, and axonal forms (CMT2), in which nerve conduction velocities are preserved
-
Until recently, most of the known CMT-related genes were associated with the CMT1 phenotype, but several genes have now been identified that are defective in patients with CMT2
-
The CMT2-associated genes encode proteins that are involved in vital cellular processes, including mitochondrial function, endosomal trafficking and RNA processing
-
Despite considerable phenotypic overlap, symptoms that are associated with specific CMT2 neuropathies are beginning to emerge; for example, CMT2B is characterized by severe sensory loss, whereas motor symptoms are more prominent in CMT2A
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Skre H (1974) Genetic and clinical aspects of CMT disease. Clin Genet 6: 78–118
Inherited Peripheral Neuropathies Mutation Database [http://www.molgen.ua.ac.be/CMTMutations]
Ben Othmane K et al. (1993) Localization of a gene (CMT2A) for autosomal dominant Charcot–Marie–Tooth disease type 2 to chromosome 1p and evidence of genetic heterogeneity. Genomics 17: 1–6
Züchner S et al. (2004) Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot–Marie–Tooth neuropathy type 2A. Nat Genet 36: 449–451
Lawson VH et al. (2005) Clinical and electrophysiologic features of CMT2A with mutations in the mitofusin 2 gene. Neurology 65: 197–204
Harding AE and Thomas PK (1980) Genetic aspects of hereditary motor and sensory neuropathy (types I and II). J Med Genet 17: 329–336
Zhu D et al. (2005) CMT with pyramidal signs is genetically heterogenous: families with and without MFN2 mutations. Neurology 65: 496–497
Rojo M et al. (2002) Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci 115: 1663–1674
Chen H and Chan DC (2004) Mitochondrial dynamics in mammals. Curr Top Dev Biol 59: 119–144
Nunnari J et al. (1997) Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Mol Biol Cell 8: 1233–1242
Bossy-Wetzel E et al. (2003) Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol 15: 706–716
Koshiba T et al. (2004) Structural basis of mitochondrial tethering by mitofusin complexes. Science 305: 858–862
Santel A and Fuller MT (2001) Control of mitochondrial morphology by a human mitofusin. J Cell Sci 114: 867–874
Pich S et al. (2005) The Charcot–Marie–Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet 14: 1405–1415
Irobi J et al. (2004) Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat Genet 36: 597–601
Evgrafov OV et al. (2004) Mutant small heat-shock protein 27 causes axonal Charcot–Marie–Tooth disease and distal hereditary motor neuropathy. Nat Genet 36: 602–606
Tang BS et al. (2004) A new locus for autosomal dominant Charcot–Marie–Tooth disease type 2 (CMT2L) maps to chromosome 12q24. Hum Genet 114: 527–533
Ganea E (2001) Chaperone-like activity of alpha-crystallin and other small heat shock proteins. Curr Protein Pept Sci 2: 205–225
Kappe G et al. (2003) The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones 8: 53–61
Mehlen P et al. (1995) Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol 154: 363–374
Carra S et al. (2005) HspB8, a small heat shock protein mutated in human neuromuscular disorders, has in vivo chaperone activity in cultured cells. Hum Mol Genet 14: 1659–1669
Preville X et al. (1999) Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp Cell Res 247: 61–78
Baxter RV et al. (2001) Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot–Marie–Tooth disease type 4A/8q21. Nat Genet 30: 21–22
Cuesta A et al. (2002) The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot–Marie–Tooth type 4A disease. Nat Genet 30: 22–25
Baxter RV et al. (2002) Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot–Marie–Tooth disease type 4A/8q21. Nat Genet 30: 21–22
Nelis E et al. (2002) Mutations in GDAP1: autosomal recessive CMT with demyelination and axonopathy. Neurology 59: 1865–1872
Senderek J et al. (2003) Mutations in the ganglioside-induced differentiation-associated protein-1 (GDAP1) gene in intermediate type autosomal recessive Charcot–Marie–Tooth neuropathy. Brain 126: 642–649
Marco A et al. (2003) Evolutionary and structural analyses of GDAP1, involved in Charcot–Marie–Tooth disease, characterize a novel class of glutathione transferase-related genes. Mol Biol Evol 21: 176–187
Pedrola L et al. (2005) GDAP1, the protein causing Charcot–Marie–Tooth disease type 4A, is expressed in neurons and is associated with mitochondria. Hum Mol Genet 14: 1087–1094
Stojkovic T et al. (2004) Vocal cord and diaphragm paralysis, as clinical features of a French family with autosomal recessive Charcot–Marie–Tooth disease, associated with a new mutation in the GDAP1 gene. Neuromuscul Disord 14: 261–264
Claramunt R et al. (2005) Genetics of Charcot–Marie–Tooth disease type 4A: mutations, inheritance, phenotypic variability, and founder effect. J Med Genet 42: 358–365
Ferri A et al. (2003) Inhibiting axon degeneration and synapse loss attenuates apoptosis and disease progression in a mouse model of motoneuron disease. Curr Biol 13: 669–673
Mersiyanova IV et al. (2000) A new variant of Charcot–Marie–Tooth disease type 2 is probably the result of a mutation in the neurofilament-light gene. Am J Hum Genet 67: 37–46
Jordanova A et al. (2003) Mutations in the neurofilament light chain gene (NEFL) cause early onset severe Charcot–Marie–Tooth disease. Brain 126: 590–597
Züchner S et al. (2004) The novel neurofilament light (NEFL) mutation Glu397Lys is associated with a clinically and morphologically heterogeneous type of Charcot–Marie–Tooth neuropathy. Neuromuscul Disord 14: 147–157
Pérez-Ollé R et al. (2005) Mutations in the neurofilament light gene linked to Charcot-Marie-Tooth disease cause defects in transport. J Neurochem 93: 861–874
Zhao C et al. (2001) Charcot–Marie–Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell 105: 587–597
Verhoeven K et al. (2003) Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot–Marie–Tooth type 2B neuropathy. Am J Hum Genet 72: 722–727
Auer-Grumbach M (2004) Hereditary sensory neuropathies. Drugs Today (Barc) 40: 385–394
Houlden H et al. (2004) A novel RAB7 mutation associated with ulcero-mutilating neuropathy 1. Ann Neurol 56: 586–590
Bottger G et al. (1996) Rab4 and Rab7 define distinct nonoverlapping endosomal compartments. J Biol Chem 271: 29191–29197
Meresse S et al. (1995) The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J Cell Sci 108: 3349–3358
Feng Y et al. (1995) Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol 131: 1435–1452
Züchner S et al. (2005) Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot–Marie–Tooth disease. Nat Genet 37: 289–294
Hinshaw JE (2000) Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol 16: 483–519
Schafer DA et al. (2002) Dynamin2 and cortactin regulate actin assembly and filament organization. Curr Biol 12: 1852–1857
Thompson HM et al. (2004) Dynamin 2 binds gamma-tubulin and participates in centrosome cohesion. Nat Cell Biol 6: 335–342
Antonellis A et al. (2003) Glycyl tRNA synthetase mutations in Charcot–Marie–Tooth disease type 2D and distal spinal muscular atrophy type V. Am J Hum Genet 72: 1293–1299
Shiba K et al. (1994) Human glycyl-tRNA synthetase: wide divergence of primary structure from bacterial counterpart and species-specific aminoacylation. J Biol Chem 269: 30049–30055
Shy ME et al. (2004) Phenotypic clustering in MPZ mutations. Brain 127: 371–384
Hattori N et al. (2003) Demyelinating and axonal features of Charcot–Marie–Tooth disease with mutations of myelin-related proteins (PMP22, MPZ and Cx32): a clinicopathological study of 205 Japanese patients. Brain 126: 134–151
Street VA et al. (2003) Mutation of a putative protein degradation gene LITAF/SIMPLE in Charcot–Marie–Tooth disease 1C. Neurology 60: 22–26
Saifi GM et al. (2005) SIMPLE mutations in Charcot–Marie–Tooth disease and the potential role of its protein product in protein degradation. Hum Mutat 25: 372–383
Sandre-Giovannoli A et al. (2002) Homozygous defects in LMNA, encoding lamin A/C nuclear-envelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot–Marie–Tooth disorder type 2) and mouse. Am J Hum Genet 70: 726–736
Chaouch M et al. (2003) The phenotypic manifestations of autosomal recessive axonal Charcot–Marie–Tooth due to a mutation in Lamin A/C gene. Neuromuscul Disord 13: 60–67
Gruenbaum Y et al. (2005) The nuclear lamina comes of age. Nat Rev Mol Cell Biol 6: 21–31
Maraldi NM et al. (2005) Laminopathies: involvement of structural nuclear proteins in the pathogenesis of an increasing number of human diseases. J Cell Physiol 203: 319–327
Harding AE and Thomas PK (1980) The clinical features of hereditary motor and sensory neuropathy types I and II. Brain 103: 259–280
Vance JM (2000) The many faces of Charcot–Marie–Tooth disease. Arch Neurol 57: 638–64055
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have licensed the testing of the MFN2 gene in CMT2 to Athena Neuroscience.
Rights and permissions
About this article
Cite this article
Züchner, S., Vance, J. Mechanisms of Disease: a molecular genetic update on hereditary axonal neuropathies. Nat Rev Neurol 2, 45–53 (2006). https://doi.org/10.1038/ncpneuro0071
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0071
This article is cited by
-
Kinesins: Motor Proteins as Novel Target for the Treatment of Chronic Pain
Molecular Neurobiology (2019)
-
Ophthalmic manifestations of inherited neurodegenerative disorders
Nature Reviews Neurology (2014)
-
TRPV4 channel activity is modulated by direct interaction of the ankyrin domain to PI(4,5)P2
Nature Communications (2014)
-
A compound heterozygous mutation in HADHB gene causes an axonal Charcot-Marie-tooth disease
BMC Medical Genetics (2013)
-
Axonal transport deficits and neurodegenerative diseases
Nature Reviews Neuroscience (2013)