Abstract
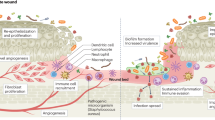
Bacteria have a basic survival strategy: to colonize surfaces and grow as biofilm communities embedded in a gel-like polysaccharide matrix. The catheterized urinary tract provides ideal conditions for the development of enormous biofilm populations. Many bacterial species colonize indwelling catheters as biofilms, inducing complications in patients' care. The most troublesome complications are the crystalline biofilms that can occlude the catheter lumen and trigger episodes of pyelonephritis and septicemia. The crystalline biofilms result from infection by urease-producing bacteria, particularly Proteus mirabilis. Urease raises the urinary pH and drives the formation of calcium phosphate and magnesium phosphate crystals in the biofilm. All types of catheter are vulnerable to encrustation by these biofilms, and clinical prevention strategies are clearly needed, as bacteria growing in the biofilm mode are resistant to antibiotics. Evidence indicates that treatment of symptomatic, catheter-associated urinary tract infection is more effective if biofilm-laden catheters are changed before antibiotic treatment is initiated. Infection with P. mirabilis exposes the many faults of currently available catheters, and plenty of scope exists for improvement in both their design and production; manufacturers should take up the challenge to improve patient outcomes.
Key Points
-
Many bacterial species colonize indwelling catheters, growing as biofilm communities embedded in a gel-like polysaccharide matrix, and induce complications in patients' care
-
The most troublesome biofilms are crystalline biofilms, generated by urease-producing bacteria, particularly Proteus mirabilis; crystalline biofilms can occlude the catheter lumen and trigger episodes of pyelonephritis and septicemia
-
Urease raises the urinary pH and drives the formation of calcium and magnesium phosphate crystals in the biofilm
-
All types of catheter, including those coated in antimicrobial agents, are vulnerable to encrustation by these biofilms, and there is a clear clinical need to develop prevention strategies
-
Bacterial cells in the biofilm mode of growth are resistant to antibiotics, and evidence indicates that treatment of symptomatic urinary tract infection is more effective if biofilmladen catheters are changed before antibiotic treatment is initiated
-
Infection with P. mirabilis exposes the many faults of current catheters; crystalline biofilms can lead to potentially disastrous consequences for patients and substantial financial implications for health-care services
-
Plenty of scope exists for improving both the design and production of catheters; manufacturers should take up this challenge
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Costerton JW et al. (1987) Bacterial biofilms in nature and disease. Annu Rev Microbiol 41: 435–464
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8: 881–890
Warren JW (1991) The catheter and urinary tract infection. Med Clin North Am 75: 481–493
Kunin CM (1997) Care of the urinary catheter. In Urinary Tract Infections: Detection, Prevention and Management, edn 5, 226–278 (Ed. Kunin CM) Baltimore: Williams & Wilkins
Stamm WE (1991) Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am J Med 91 (Suppl 3B): 65S–71S
Tambyah PA et al. (1999) A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin Proc 74: 131–136
Matsukawa M et al. (2005) Bacterial colonization on intraluminal surface of urethral catheter. Urology 65: 440–444
Clayton CL et al. (1982) Some observations on urinary tract infections in patients undergoing long-term bladder catheterization. J Hosp Infect 3: 39–47
Warren JW et al. (1982) Cephalexin for susceptible bacteriuria in afebrile, long-term catheterized patients. JAMA 248: 454–458
Saint S and Chenoweth CE (2003) Biofilms and catheter-associated urinary tract infections. Infect Dis Clin North Am 17: 411–432
Trautner BW and Darouiche RO (2004) Role of biofilm in catheter-associated urinary tract infection. Am J Infect Control 32: 177–183
Tenke P et al. (2008) European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. Int J Antimicrob Agents 31 (Suppl 1): S68–S78
Ganderton L et al. (1992) Scanning electron microscopy of bacterial biofilms on indwelling bladder catheters. Eur J Clin Microbiol Infect Dis 11: 789–796
Morris NS et al. (1999) The development of bacterial biofilms on indwelling urethral catheters. World J Urol 17: 345–350
Liedl B (2001) Catheter-associated urinary tract infections. Curr Opin Urol 11: 75–79
Ohkawa M et al. (1990) Bacterial and crystal adherence to the surfaces of indwelling urethral catheters. J Urol 143: 717–721
Macleod SM and Stickler DJ (2007) Species interactions in mixed-community crystalline biofilms on urinary catheters. J Med Microbiol 56: 1549–1557
Getliffe KA and Mulhall AB (1991) The encrustation of indwelling catheters. Br J Urol 67: 337–341
Stickler DJ and Zimakoff J (1994) Complications of urinary tract infections associated with devices used for long-term bladder management. J Hosp Infect 28: 177–194
Kunin CM (1987) Care of the urinary catheter. In Detection, Prevention and Management of Urinary Tract Infections, edn 4, 245–298 (Ed. Kunin CM) Philadelphia: Lea & Febiger
Cools HJ and Van der Meer JW (1986) Restriction of long-term indwelling urethral catheterisation in the elderly. Br J Urol 58: 683–688
Getliffe KA (1994) The characteristics and management of patients with recurrent blockage of long-term urinary catheters. J Adv Nurs 20: 140–149
Kohler-Ockmore J and Feneley RC (1996) Long-term catheterization of the bladder: prevalence and morbidity. Br J Urol 77: 347–351
Hedelin H et al. (1984) The composition of catheter encrustations, including the effects of allopurinol treatment. Br J Urol 56: 250–254
Cox AJ and Hukins DW (1989) Morphology of mineral deposits on encrusted urinary catheters investigated by scanning electron microscopy. J Urol 142: 1347–1350
Cox AJ et al. (1989) Infection of catheterised patients: bacterial colonisation of encrusted Foley catheters shown by scanning electron microscopy. Urol Res 17: 349–352
Stickler D et al. (1993) Proteus mirabilis biofilms and the encrustation of urethral catheters. Urol Res 21: 407–411
McLean RJ et al. (1996) Biofilm mediated calculus formation in the urinary tract. Cells Mater 6: 165–174
Mobley HL and Warren JW (1987) Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol 25: 2216–2217
Kunin CM (1989) Blockage of urinary catheters: role of microorganisms and constituents of the urine on formation of encrustations. J Clin Epidemiol 42: 835–842
Jones BD and Mobley HL (1987) Genetic and biochemical diversity of ureases of Proteus, Providencia, and Morganella species isolated from urinary tract infection. Infect Immun 55: 2198–2203
Stickler D et al. (1998) Studies on the formation of crystalline bacterial biofilms on urethral catheters. Eur J Clin Microbiol Infect Dis 17: 649–652
Mobley HT (1996) Virulence of Proteus mirabilis . In Urinary tract infections: molecular pathogenesis and clinical management, 245–270 (Eds Mobley HL and Warren JW) Washington DC: ASM Press
Sabbuba NA et al. (2003) Molecular epidemiology of Proteus mirabilis infections of the catheterized urinary tract. J Clin Microbiol 41: 4961–4965
Sabbuba NA et al. (2004) Genotyping demonstrates that the strains of Proteus mirabilis from bladder stones and catheter encrustations of patients undergoing long-term bladder catheterization are identical. J Urol 171: 1925–1928
Mathur S et al. (2005) Genotyping of urinary and fecal Proteus mirabilis isolates from individuals with long-term urinary catheters. Eur J Clin Microbiol Infect Dis 24: 643–644
Morris NS et al. (1997) Which indwelling urethral catheters resist encrustation by Proteus mirabilis? biofilms Br J Urol 80: 58–63
Stickler DJ and Sabbuba NA (2007) Antimicrobial catheters. In Disinfection and Decontamination Principles, Applications and Related Issues, 415–458 (Ed. Manivannan G) Boca Raton: CRC Press
Capewell AE and Morris SL (1993) Audit of catheter management provided by District Nurses and Continence Advisors. Br J Urol 71: 259–264
Stickler DJ (1996) Biofilms, catheters and urinary tract infections. Eur Urol Update Series 5: 1–8
Donlan RM and Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15: 167–193
Ong CL et al. (2008) Identification of type 3 fimbriae in uropathogenic Escherichia coli reveals a role in biofilm formation. J Bacteriol 190: 1054–1063
Rocha SP et al. (2007) Fimbriae of uropathogenic Proteus mirabilis . FEMS Immunol Med Microbiol 51: 1–7
Jacobsen SM et al. (2008) Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis . Clin Microbiol Rev 21: 26–59
Downer A et al. (2003) Polymer surface properties and their effect on the adhesion of Proteus mirabilis . Proc Inst Mech Eng [H] 217: 279–289
Stickler DJ et al. (2006) Observations on the adherence of Proteus mirabilis onto polymer surfaces. J Appl Microbiol 100: 1028–1033
Jones BV et al. (2005) Role of swarming in the formation of crystalline Proteus mirabilis biofilms on urinary catheters. J Med Microbiol 54: 807–813
Cox AJ (1990) Comparison of catheter surface morphologies. Br J Urol 65: 55–60
Stickler D et al. (2003) Why are Foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm> Urol Res 31: 306–311
Stickler DJ and Morgan SD (2008) Observations on the development of the crystalline bacterial biofilms that encrust and block Foley catheters. J Hosp Infect 69: 350–360
Brisset L et al. (1996) In vivo and in vitro analysis of the ability of urinary catheter to microbial colonization. Pathol Biol (Paris) 44: 397–404
Mathur S et al. (2006) Prospective study of individuals with long-term urinary catheters colonized with Proteus species. BJU Int 97: 121–128
Choong S et al. (2001) Catheter associated urinary tract infection and encrustation. Int J Antimicrob Agents 17: 305–310
Mathur S et al. (2006) Factors affecting crystal precipitation from urine in individuals with long-term urinary catheters colonized with urease-positive bacterial species. Urol Res 34: 173–177
Suller MT et al. (2005) Factors modulating the pH at which calcium and magnesium phosphates precipitate from human urine. Urol Res 33: 254–260
Stickler DJ and Morgan SD (2006) Modulation of crystalline Proteus mirabilis biofilm development on urinary catheters. J Med Microbiol 55: 489–494
Burr RG and Nuseibeh IM (1997) Urinary catheter blockage depends on urine pH, calcium and rate of flow. Spinal Cord 35: 521–525
Chakravarti A et al. (2005) An electrified catheter to resist encrustation by Proteus mirabilis biofilm. J Urol 174: 1129–1132
Bibby JM et al. (1995) Feasibility of preventing encrustation of urinary catheters. Cells Mater 2: 183–195
Stickler DJ (2002) Susceptibility of antibiotic-resistant Gram-negative bacteria to biocides: a perspective from the study of catheter biofilms. J Appl Microbiol 92 (Suppl): 163S–170S
Williams GJ and Stickler DJ (2007) Some observations on the diffusion of antimicrobial agents through the retention balloons of Foley catheters. J Urol 178: 697–701
Stickler DJ et al. (2003) Control of encrustation and blockage of Foley catheters. Lancet 361: 1435–1437
Jones GL et al. (2005) A strategy for the control of catheter blockage by crystalline Proteus mirabilis biofilm using the antibacterial agent triclosan. Eur Urol 48: 838–845
Jones GL et al. (2006) Effect of triclosan on the development of bacterial biofilms by urinary tract pathogens on urinary catheters. J Antimicrob Chemother 57: 266–272
Stickler DJ and Jones GL (2008) Reduced susceptibility of Proteus mirabilis to triclosan. Antimicrob Agents Chemother 52: 991–994
Kunin CM (1988) Can we build a better urinary catheter. N Engl J Med 319: 365–366
Lewis K (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45: 999–1007
Tenney JH and Warren JW (1988) Bacteriuria in women with long-term catheters: paired comparison of indwelling and replacement catheters. J Infect Dis 157: 199–202
Ramsay JW et al. (1989) Biofilms, bacteria and bladder catheters. A clinical study. Br J Urol 64: 395–398
Raz R et al. (2000) Chronic indwelling catheter replacement before antimicrobial therapy for symptomatic urinary tract infection. J Urol 164: 1254–1258
Norberg B et al. (1983) The spontaneous variation of catheter life in long-stay geriatric inpatients with indwelling catheters. Gerontology 29: 332–335
Stickler DJ et al. (2006) A clinical assessment of the performance of a sensor to detect crystalline biofilm formation on indwelling bladder catheters? BJU Int 98: 1244–1249
Acknowledgements
The author would like to thank Dr Rob Broomfield, Dr Sheridan Morgan and Dr Rob Young, who are members of Dr Stickler's research team, for providing the previously unpublished images shown in this Review. Charles P Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Stickler, D. Bacterial biofilms in patients with indwelling urinary catheters. Nat Rev Urol 5, 598–608 (2008). https://doi.org/10.1038/ncpuro1231
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro1231
This article is cited by
-
Detection of microbial biofilms inside the lumen of ureteral stents: two case reports
Journal of Medical Case Reports (2023)
-
Rectifying disorder of extracellular matrix to suppress urethral stricture by protein nanofilm-controlled drug delivery from urinary catheter
Nature Communications (2023)
-
Uropathogenic bacteria and deductive genomics towards antimicrobial resistance, virulence, and potential drug targets
International Microbiology (2023)
-
A standard 96-well based high throughput microfluidic perfusion biofilm reactor for in situ optical analysis
Biomedical Microdevices (2023)
-
Clonal relationship, virulence genes, and antimicrobial resistance of Morganella morganii isolated from community-acquired infections and hospitalized patients: a neglected opportunistic pathogen
International Microbiology (2023)