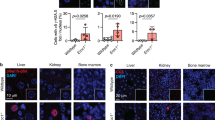
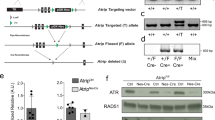
Abstract
Although DNA damage is considered a driving force for aging, the nature of the damage that arises endogenously remains unclear. Replicative stress, a source of endogenous DNA damage, is prevented primarily by the ATR kinase. We have developed a mouse model of Seckel syndrome characterized by a severe deficiency in ATR. Seckel mice show high levels of replicative stress during embryogenesis, when proliferation is widespread, but this is reduced to marginal amounts in postnatal life. In spite of this decrease, adult Seckel mice show accelerated aging, which is further aggravated in the absence of p53. Together, these results support a model whereby replicative stress, particularly in utero, contributes to the onset of aging in postnatal life, and this is balanced by the replicative stress–limiting role of the checkpoint proteins ATR and p53.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lombard, D.B. et al. DNA repair, genome stability, and aging. Cell 120, 497–512 (2005).
Sedelnikova, O.A. et al. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 6, 168–170 (2004).
Rossi, D.J. et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447, 725–729 (2007).
Schumacher, B., Garinis, G.A. & Hoeijmakers, J.H. Age to survive: DNA damage and aging. Trends Genet. 24, 77–85 (2008).
Harper, J.W. & Elledge, S.J. The DNA damage response: ten years after. Mol. Cell 28, 739–745 (2007).
Xu, Y. & Baltimore, D. Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev. 10, 2401–2410 (1996).
Elson, A. et al. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc. Natl. Acad. Sci. USA 93, 13084–13089 (1996).
Barlow, C. et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86, 159–171 (1996).
Brown, E.J. & Baltimore, D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 17, 615–628 (2003).
de Klein, A. et al. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr. Biol. 10, 479–482 (2000).
O'Driscoll, M., Ruiz-Perez, V.L., Woods, C.G., Jeggo, P.A. & Goodship, J.A. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat. Genet. 33, 497–501 (2003).
Seckel, H. Bird-Headed Dwarfs: Studies in Developmental Anthropology Including Human Proportions (Karger, Basel, Switzerland, 1960).
Shanske, A., Caride, D.G., Menasse-Palmer, L., Bogdanow, A. & Marion, R.W. Central nervous system anomalies in Seckel syndrome: report of a new family and review of the literature. Am. J. Med. Genet. 70, 155–158 (1997).
Ruzankina, Y. et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1, 113–126 (2007).
Butler, M.G., Hall, B.D., Maclean, R.N. & Lozzio, C.B. Do some patients with Seckel syndrome have hematological problems and/or chromosome breakage? Am. J. Med. Genet. 27, 645–649 (1987).
Rossi, D.J., Jamieson, C.H. & Weissman, I.L. Stems cells and the pathways to aging and cancer. Cell 132, 681–696 (2008).
Rosen, C.J. & Bouxsein, M.L. Mechanisms of disease: is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol. 2, 35–43 (2006).
Morrison, S.J., Wandycz, A.M., Akashi, K., Globerson, A. & Weissman, I.L. The aging of hematopoietic stem cells. Nat. Med. 2, 1011–1016 (1996).
Sudo, K., Ema, H., Morita, Y. & Nakauchi, H. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 192, 1273–1280 (2000).
Bartke, A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology 146, 3718–3723 (2005).
Lombardi, G., Di Somma, C., Rota, F. & Colao, A. Associated hormonal decline in aging: is there a role for GH therapy in aging men? J. Endocrinol. Invest. 28, 99–108 (2005).
van der Pluijm, I. et al. Impaired genome maintenance suppresses the growth hormone–insulin-like growth factor 1 axis in mice with Cockayne syndrome. PLoS Biol. 5, e2 (2007).
Niedernhofer, L.J. et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444, 1038–1043 (2006).
Cha, R.S. & Kleckner, N. ATR homolog Mec1 promotes fork progression, thus averting breaks in replication slow zones. Science 297, 602–606 (2002).
Sogo, J.M., Lopes, M. & Foiani, M. Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science 297, 599–602 (2002).
Stiff, T. et al. Nbs1 is required for ATR-dependent phosphorylation events. EMBO J. 24, 199–208 (2005).
Casper, A.M., Nghiem, P., Arlt, M.F. & Glover, T.W. ATR regulates fragile site stability. Cell 111, 779–789 (2002).
Casper, A.M., Durkin, S.G., Arlt, M.F. & Glover, T.W. Chromosomal instability at common fragile sites in Seckel syndrome. Am. J. Hum. Genet. 75, 654–660 (2004).
Bobabilla-Morales, L. et al. Chromosome instability induced in vitro with mitomycin C in five Seckel syndrome patients. Am. J. Med. Genet. A. 123A, 148–152 (2003).
Syrrou, M., Georgiou, I., Paschopoulos, M. & Lolis, D. Seckel syndrome in a family with three affected children and hematological manifestations associated with chromosome instability. Genet. Couns. 6, 37–41 (1995).
Arnold, S.R., Spicer, D., Kouseff, B., Lacson, A. & Gilbert-Barness, E. Seckel-like syndrome in three siblings. Pediatr. Dev. Pathol. 2, 180–187 (1999).
Boscherini, B. et al. Intrauterine growth retardation. A report of two cases with bird-headed appearance, skeletal changes and peripheral GH resistance. Eur. J. Pediatr. 137, 237–242 (1981).
Fathizadeh, A., Soltani, K., Medenica, M. & Lorincz, A.L. Pigmentary changes in Seckel's syndrome. J. Am. Acad. Dermatol. 1, 52–54 (1979).
Griffith, E. et al. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat. Genet. 40, 232–236 (2008).
Goodship, J. et al. Autozygosity mapping of a Seckel syndrome locus to chromosome 3q22. 1-q24. Am. J. Hum. Genet. 67, 498–503 (2000).
Fowden, A.L., Giussani, D.A. & Forhead, A.J. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 21, 29–37 (2006).
Fowden, A.L., Forhead, A.J., Coan, P.M. & Burton, G.J. The placenta and intrauterine programming. J. Neuroendocrinol. 20, 439–450 (2008).
Barker, D. The fetal origins of adult disease. Proc. R. Soc. Lond. B 262, 37–43 (1995).
Murphy, D.P. Ovarian radiation—its effect on the health of subsequent children. Review of the literature, experimental and clinical, with a report of 320 human pregnancies. Surg. Gynecol. Obstet. 47, 201–215 (1928).
Schmidt, S.L. & Lent, R. Effects of prenatal irradiation on the development of cerebral cortex and corpus callosum of the mouse. J. Comp. Neurol. 264, 193–204 (1987).
Rodier, F., Campisi, J. & Bhaumik, D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 35, 7475–7484 (2007).
el-Deiry, W.S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Sidi, S. et al. Chk1 suppresses a caspase-2 apoptotic response to DNA damage that bypasses p53, Bcl-2, and caspase-3. Cell 133, 864–877 (2008).
Wang, Q. et al. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J. Natl. Cancer Inst. 88, 956–965 (1996).
Viale, A. et al. Cell-cycle restriction limits DNA damage and maintains self-renewal of leukaemia stem cells. Nature 457, 51–56 (2009).
Matheu, A. et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature 448, 375–379 (2007).
Brown, E.J. & Baltimore, D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 14, 397–402 (2000).
Liu, Q. et al. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 14, 1448–1459 (2000).
Jacks, T. et al. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4, 1–7 (1994).
Murga, M. et al. Global chromatin compaction limits the strength of the DNA damage response. J. Cell Biol. 178, 1101–1108 (2007).
Acknowledgements
We thank M. Serrano and A. Ramiro for critical comments on the manuscript. We also thank S.P. Jackson for his help with the PIKK inhibitors and A. Garcia for cytometry. M.M. is supported by a Ramón y Cajal contract from the Spanish Ministry of Science (RYC-2003-002731) and from a grant from Fondo de Investigaciones Sanitarias (PI080220). Work in O.F.-C.'s laboratory is supported by grants from the Spanish Ministry of Science (RYC-2003-002731, CSD2007-00017 and SAF2008-01596), European Molecular Biology Organization Young Investigator Programme, European Research Council (ERC-210520) and Epigenome Network of Excellence.
Author information
Authors and Affiliations
Contributions
O.F.-C. designed the study and experiments and wrote the paper. M.M. performed most of the experiments presented. M.F.M. and R.S. helped in the analysis of Seckel MEFs and embryos. S.B. and A.N. performed HSC and chromosomal breakage analyses. F.M. helped with the whole body Imaging. M.C. helped with the pathology. Y.L. and P.J.M. performed the analyses of the brains.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 3958 kb)
Rights and permissions
About this article
Cite this article
Murga, M., Bunting, S., Montaña, M. et al. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat Genet 41, 891–898 (2009). https://doi.org/10.1038/ng.420
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.420
This article is cited by
-
The role of gut microbiota in human metabolism and inflammatory diseases: a focus on elderly individuals
Annals of Microbiology (2024)
-
EYA4 promotes breast cancer progression and metastasis through its role in replication stress avoidance
Molecular Cancer (2023)
-
YTHDC1 delays cellular senescence and pulmonary fibrosis by activating ATR in an m6A-independent manner
The EMBO Journal (2023)
-
LAP2α preserves genome integrity through assisting RPA deposition on damaged chromatin
Genome Biology (2022)
-
ATR regulates neuronal activity by modulating presynaptic firing
Nature Communications (2021)