Abstract
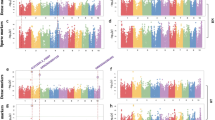
US maize yield has increased eight-fold in the past 80 years, with half of the gain attributed to selection by breeders. During this time, changes in maize leaf angle and size have altered plant architecture, allowing more efficient light capture as planting density has increased. Through a genome-wide association study (GWAS) of the maize nested association mapping panel, we determined the genetic basis of important leaf architecture traits and identified some of the key genes. Overall, we demonstrate that the genetic architecture of the leaf traits is dominated by small effects, with little epistasis, environmental interaction or pleiotropy. In particular, GWAS results show that variations at the liguleless genes have contributed to more upright leaves. These results demonstrate that the use of GWAS with specially designed mapping populations is effective in uncovering the basis of key agronomic traits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Duvick, D.N. Genetic progress in yield of United States maize (Zea mays L.). Maydica 50, 193–202 (2005).
Pendleton, J.W., Smith, G.E., Winter, S.R. & Johnston, T.J. Field investigation of the relationships of leaf angle in corn (Zea mays L.) to grain yield and apparent photosynthesis. Agron. J. 60, 422–424 (1968).
Lambert, R.J. & Johnson, R.R. Leaf angle, tassel morphology, and the performance of maize hybrids. Crop Sci. 18, 499–502 (1978).
Duncan, W.G. Leaf angle, leaf area, and canopy photosynthesis. Crop Sci. 11, 482–485 (1971).
Pepper, G.E., Pearce, R.B. & Mock, J.J. Leaf orientation and yield of maize. Crop Sci. 17, 883–886 (1977).
Sinclair, T.R. & Sheehy, J.E. Erect leaves and photosynthesis in rice. Science 283, 1455 (1999).
Gore, M.A. et al. A first-generation haplotype map of maize. Science 326, 1115–1117 (2009).
McMullen, M.D. et al. Genetic properties of the maize nested association mapping population. Science 325, 737–740 (2009).
Buckler, E.S. et al. The genetic architecture of maize flowering time. Science 325, 714–718 (2009).
Laurie, C.C. et al. The genetic architecture of response to long-term artificial selection for oil concentration in the maize kernel. Genetics 168, 2141–2155 (2004).
Flint, J. & Mackay, T.F. Genetic architecture of quantitative traits in mice, flies, and humans. Genome Res. 19, 723–733 (2009).
Koornneef, M., Alonso-Blanco, C. & Vreugdenhil, D. Naturally occurring genetic variation in Arabidopsis thaliana. Annu. Rev. Plant Biol. 55, 141–172 (2004).
Takahashi, Y., Teshima, K.M., Yokoi, S., Innan, H. & Shimamoto, K. Variations in Hd1 proteins, Hd3a promoters, and Ehd1 expression levels contribute to diversity of flowering time in cultivated rice. Proc. Natl. Acad. Sci. USA 106, 4555–4560 (2009).
Turner, A., Beales, J., Faure, S., Dunford, R.P. & Laurie, D.A. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310, 1031–1034 (2005).
Rice, W.R. Analyzing tables of statistical tests. Evolution 43, 223–225 (1989).
Chopra, S., Athma, P., Li, X.G. & Peterson, T. A maize Myb homolog is encoded by a multicopy gene complex. Mol. Gen. Genet. 260, 372–380 (1998).
Palaisa, K.A., Morgante, M., Williams, M. & Rafalski, A. Contrasting effects of selection on sequence diversity and linkage disequilibrium at two phytoene synthase loci. Plant Cell 15, 1795–1806 (2003).
Valdar, W. et al. Genome-wide genetic association of complex traits in heterogeneous stock mice. Nat. Genet. 38, 879–887 (2006).
Huang, G.J. et al. High resolution mapping of expression QTLs in heterogeneous stock mice in multiple tissues. Genome Res. 19, 1133–1140 (2009).
Valdar, W., Holmes, C.C., Mott, R. & Flint, J. Mapping in structured populations by resample model averaging. Genetics 182, 1263–1277 (2009).
Dickson, S.P., Wang, K., Krantz, I., Hakonarson, H. & Goldstein, D.B. Rare variants create synthetic genome-wide associations. PLoS Biol. 8, e1000294 (2010).
Moreno, M.A., Harper, L.C., Krueger, R.W., Dellaporta, S.L. & Freeling, M. liguleless1 encodes a nuclear-localized protein required for induction of ligules and auricles during maize leaf organogenesis. Genes Dev. 11, 616–628 (1997).
Walsh, J., Waters, C.A. & Freeling, M. The maize gene liguleless2 encodes a basic leucine zipper protein involved in the establishment of the leaf blade-sheath boundary. Genes Dev. 12, 208–218 (1998).
Clark, R.M., Wagler, T.N., Quijada, P. & Doebley, J. A distant upstream enhancer at the maize domestication gene tb1 has pleiotropic effects on plant and inflorescent architecture. Nat. Genet. 38, 594–597 (2006).
Salvi, S. et al. Conserved noncoding genomic sequences associated with a flowering-time quantitative trait locus in maize. Proc. Natl. Acad. Sci. USA 104, 11376–11381 (2007).
Lettice, L.A. et al. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum. Mol. Genet. 12, 1725–1735 (2003).
Flint-Garcia, S.A. et al. Maize association population: a high-resolution platform for quantitative trait locus dissection. Plant J. 44, 1054–1064 (2005).
Schork, N.J., Murray, S.S., Frazer, K.A. & Topol, E.J. Common vs. rare allele hypotheses for complex diseases. Curr. Opin. Genet. Dev. 19, 212–219 (2009).
Lai, J. et al. Genome-wide patterns of genetic variation among elite maize inbred lines. Nat. Genet. 42, 1027–1030 (2010).
Swanson-Wagner, R.A. et al. Pervasive gene content variation and copy number variation in maize and its undomesticated progenitor. Genome Res. 20, 1689–1699 (2010).
Lee, M. et al. Expanding the genetic map of maize with the intermated B73 x Mo17 (IBM) population. Plant Mol. Biol. 48, 453–461 (2002).
Mickelson, S.M., Stuber, C.S., Senior, L. & Kaeppler, S.M. Quantitative trait loci controlling leaf and tassel traits in a B73 x Mo17 population of maize. Crop Sci. 42, 1902–1909 (2002).
Gilmour, A.R., Gogel, B.J., Cullis, B.R. & Thompson, R. ASReml User Guide: Release 2.0. (VSN International Ltd, Hemel Hempstead, UK, 2006).
Box, G.E.P. & Cox, D.R. An analysis of transformations. J. R. Stat. Soc., B 26, 211–252 (1964).
Scheet, P. & Stephens, M. A fast and flexible statistical model for large-scale population genotype data: applications to inferring missing genotypes and haplotypic phase. Am. J. Hum. Genet. 78, 629–644 (2006).
Schnable, P.S. et al. The B73 maize genome: complexity, diversity, and dynamics. Science 326, 1112–1115 (2009).
Acknowledgements
We thank L. Rigamer Lirette and S. Myles for editing the manuscript. This work was supported by US National Science Foundation grants (DBI-0820619, 0321467, 0703908 and 0638566) and USDA-ARS.
Author information
Authors and Affiliations
Contributions
F.T. and P.J. Bradbury contributed equally to this work. M.D.M., J.B.H. and E.S.B. contributed to the study design. S.F.-G., T.R.R., M.D.M., J.B.H. and E.S.B. collected phenotypes. F.T., P.J. Bradbury, P.J. Brown, H.H., Q.S. and E.S.B. carried out analysis. F.T., P.J. Bradbury and E.S.B. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Figures 1–7 and Supplementary Tables 1–14 (PDF 2033 kb)
Rights and permissions
About this article
Cite this article
Tian, F., Bradbury, P., Brown, P. et al. Genome-wide association study of leaf architecture in the maize nested association mapping population. Nat Genet 43, 159–162 (2011). https://doi.org/10.1038/ng.746
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.746
This article is cited by
-
Genomic variation and candidate genes dissect quality and yield traits in Boehmeria nivea (L.) Gaudich
Cellulose (2024)
-
A potential candidate gene associated with the angles of the ear leaf and the second leaf above the ear leaf in maize
BMC Plant Biology (2023)
-
Genetic variation among elite inbred lines suggests potential to breed for BNI-capacity in maize
Scientific Reports (2023)
-
Genome-wide association studies: an intuitive solution for SNP identification and gene mapping in trees
Functional & Integrative Genomics (2023)
-
Nested association mapping population in crops: current status and future prospects
Journal of Crop Science and Biotechnology (2023)