Abstract
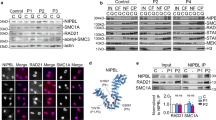
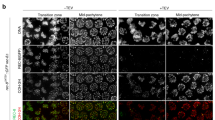
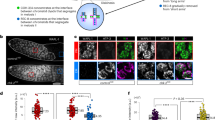
Mitotic chromosome segregation is facilitated by the cohesin complex, which maintains physical connections between sister chromatids until anaphase. Meiotic cell division is considerably more complex, as cohesion must be released sequentially to facilitate orderly segregation of chromosomes at both meiosis I and meiosis II. This necessitates meiosis-specific cohesin components; recent studies in rodents suggest that these influence chromosome behavior during both cell division and meiotic prophase1,2. To elucidate the role of the meiosis-specific cohesin SMC1β (encoded by Smc1l2) in oogenesis, we carried out meiotic studies of female SMC1β-deficient mice. Our results provide the first direct evidence that SMC1β acts as a chiasma binder in mammals, stabilizing sites of exchange until anaphase. Additionally, our observations support the hypothesis that deficient cohesion is an underlying cause of human age-related aneuploidy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Eijpe, M., Offenberg, H., Jessberger, R., Revenkova, E. & Heyting, C. Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1beta and SMC3. J. Cell Biol. 160, 657–670 (2003).
Revenkova, E. et al. Cohesin SMC1β is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat. Cell Biol. 6, 555–562 (2004).
Uhlmann, F. The mechanism of sister chromatid cohesion. Exp. Cell Res. 296, 80–85 (2004).
Gruber, S., Haering, C.H. & Nasmyth, K. Chromosomal cohesin forms a ring. Cell 112, 765–777 (2003).
Nasmyth, K., Peters, J.M. & Uhlmann, F. Splitting the chromosome: cutting the ties that bind sister chromatids. Science 288, 1379–1385 (2000).
Petronczki, M., Siomos, M.F. & Nasmyth, K. Un menage a quatre: the molecular biology of chromosome segregation in meiosis. Cell 112, 423–440 (2003).
Parisi, S. et al. Rec8p, a meiotic recombination and sister chromatid cohesion phosphoprotein of the Rad21p family conserved from fission yeast to humans. Mol. Cell. Biol. 19, 3515–3528 (1999).
Prieto, I. et al. Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat. Cell Biol. 3, 761–766 (2001).
Revenkova, E., Eijpe, M., Heyting, C., Gross, B. & Jessberger, R. Novel meiosis-specific isoform of mammalian SMC1. Mol. Cell. Biol. 21, 6984–6998 (2001).
Lamb, N.E. et al. Susceptible chiasmate configurations of chromosome 21 predispose to non-disjunction in both maternal meiosis I and meiosis II. Nat. Genet. 14, 400–405 (1996).
Lynn, A., Ashley, T. & Hassold, T. Variation in human meiotic recombination. Annu. Rev. Genomics Hum. Genet. 5, 317–349 (2004).
Chan, R.C. et al. Chromosome cohesion is regulated by a clock gene paralogue TIM-1. Nature 423, 1002–1009 (2003).
Lynn, A. et al. Covariation of synaptonemal complex length and mammalian meiotic exchange rates. Science 296, 2222–2225 (2002).
Sun, F. et al. Human male recombination maps for individual chromosomes. Am. J. Hum. Genet. 74, 521–531 (2004).
Maguire, M.P. Letter: The need for a chiasma binder. J. Theor. Biol. 48, 485–487 (1974).
Bickel, S.E., Orr-Weaver, T.L. & Balicky, E.M. The sister-chromatid cohesion protein ORD is required for chiasma maintenance in Drosophila oocytes. Curr. Biol. 12, 925–929 (2002).
Buonomo, S.B. et al. Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell 103, 387–398 (2000).
Pellestor, F., Andreo, B., Arnal, F., Humeau, C. & Demaille, J. Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum. Genet. 112, 195–203 (2003).
Wolstenholme, J. & Angell, R.R. Maternal age and trisomy–a unifying mechanism of formation. Chromosoma 109, 435–438 (2000).
Angell, R. First-meiotic-division nondisjunction in human oocytes. Am. J. Hum. Genet. 61, 23–32 (1997).
Henderson, S.A. & Edwards, R.G. Chiasma frequency and maternal age in mammals. Nature 218, 22–28 (1968).
Polani, P.E. & Jagiello, G.M. Chiasmata, meiotic univalents, and age in relation to aneuploid imbalance in mice. Cytogenet. Cell Genet. 16, 505–529 (1976).
Haering, C.H. et al. Structure and stability of cohesin's Smc1-kleisin interaction. Mol. Cell 15, 951–964 (2004).
Prieto, I. et al. Cohesin component dynamics during meiotic prophase I in mammalian oocytes. Chromosome Res. 12, 197–213 (2004).
Kouznetsova, A., Novak, I., Jessberger, R. & Hoog, C. SYCP2 and SYCP3 are required for cohesin core integrity at diplotene but not for centromere cohesion at the first meiotic division. J. Cell Sci. 118, 2271–2278 (2005).
Hogan, B., Constantini, F. & Lacey, E. Manipulating the Mouse Embryo: A Laboratory Manual (Cold Spring Harbor Laboratory, New York, 1986).
Tarkowski, A.K. An air-drying method for chromosome preparations from mouse eggs. Cytogenetics 5, 394–400 (1966).
Hodges, C.A. & Hunt, P.A. Simultaneous analysis of chromosomes and chromosome-associated proteins in mammalian oocytes and embryos. Chromosoma 111, 165–169 (2002).
Rasmussen, R. Quantification on the LightCycler instrument. in Rapid Cycle Real-Time PCR: Methods and Applications (eds. Meuer, S., Wittwer, C. & Nakagawara, K.) 21–34 (Springer, Heidelberg, Germany, 2001).
Acknowledgements
We thank T. Ashley and S. Varmuza for supplying antibodies for these studies. This work was supported by grants from the US National Institutes of Health (P.A.H., T.J.H. and R.J.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Scoring of recombination events (MLH1 foci and chiasmata) in wildtype and Smc1β−/− oocytes. (PDF 334 kb)
Supplementary Fig. 2
Example of methodology used to compare relative position of DNA sequences on the SC and on condensed chromosomes. (PDF 84 kb)
Supplementary Fig. 3
Quantitative RT-PCR of Smc1β RNA in different aged wildtype oocytes. (PDF 155 kb)
Supplementary Table 1
Placement of MLH1 foci and chiasmata with respect to BAC probes on the X chromosome. (PDF 13 kb)
Rights and permissions
About this article
Cite this article
Hodges, C., Revenkova, E., Jessberger, R. et al. SMC1β-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet 37, 1351–1355 (2005). https://doi.org/10.1038/ng1672
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1672
This article is cited by
-
Integrated analyses of miRNA-mRNA expression profiles of ovaries reveal the crucial interaction networks that regulate the prolificacy of goats in the follicular phase
BMC Genomics (2021)
-
Molecular basis of reproductive senescence: insights from model organisms
Journal of Assisted Reproduction and Genetics (2021)
-
Emerging themes in cohesin cancer biology
Nature Reviews Cancer (2020)
-
Increased levels of superoxide dismutase suppress meiotic segregation errors in aging oocytes
Chromosoma (2019)
-
Maternal obesity enhances oocyte chromosome abnormalities associated with aging
Chromosoma (2019)