Abstract
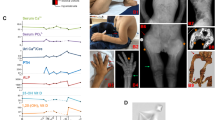
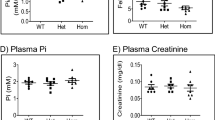
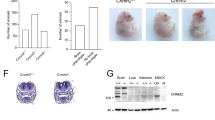
Magnesium is an essential ion involved in many biochemical and physiological processes. Homeostasis of magnesium levels is tightly regulated and depends on the balance between intestinal absorption and renal excretion. However, little is known about specific proteins mediating transepithelial magnesium transport. Using a positional candidate gene approach, we identified mutations in TRPM6 (also known as CHAK2), encoding TRPM6, in autosomal-recessive hypomagnesemia with secondary hypocalcemia (HSH, OMIM 602014)1,2, previously mapped to chromosome 9q22 (ref. 3). The TRPM6 protein is a new member of the long transient receptor potential channel (TRPM) family4 and is highly similar to TRPM7 (also known as TRP-PLIK), a bifunctional protein that combines calcium- and magnesium-permeable cation channel properties with protein kinase activity5,6,7. TRPM6 is expressed in intestinal epithelia and kidney tubules. These findings indicate that TRPM6 is crucial for magnesium homeostasis and implicate a TRPM family member in human disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Paunier, L., Radde, I.C., Kooh, S.W., Conen, P.E. & Fraser, D. Primary hypomagnesemia with secondary hypocalcemia in an infant. Pediatrics 41, 385–402 (1968).
Shalev, H., Phillip, M., Galil, A., Carmi, R. & Landau, D. Clinical presentation and outcome in primary familial hypomagnesaemia. Arch. Dis. Child. 78, 127–130 (1998).
Walder, R.Y. et al. Familial hypomagnesemia maps to chromosome 9q, not to the X chromosome: genetic linkage mapping and analysis of a balanced translocation breakpoint. Hum. Mol. Genet. 6, 1491–1497 (1997).
Ryazanova, L.V., Pavur, K.S., Petrov, A.N., Dorovkov, M.V. & Ryazanov, A.G. Novel type of signaling molecules: protein kinases covalently linked with ion channels. Mol. Biol. 35, 271–283 (2001).
Nadler, M.J. et al. LTRPC7 is a Mg-ATP-regulated divalent cation channel required for cell viability. Nature 411, 590–595 (2001).
Runnels, L.W., Yue, L. & Clapham, D.E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047 (2001).
Ryazanov, A.G. et al. Identification of a new class of protein kinases represented by eukaryotic elongation factor-2 kinase. Proc. Natl Acad. Sci. USA 94, 4884–4889 (1997).
Anast, C.S., Mohs, J.M., Kaplan, S.L. & Burns, T.W. Evidence for parathyroid failure in magnesium deficiency. Science 177, 606–608 (1972).
Milla, P.J., Aggett, P.J., Wolff, O.H. & Harries, J.T. Studies in primary hypomagnesaemia: evidence for defective carrier-mediated small intestinal transport of magnesium. Gut 20, 1028–1033 (1979).
Matzkin, H., Lotan, D. & Boichis, H. Primary hypomagnesemia with a probable double magnesium transport defect. Nephron 52, 83–86 (1989).
Cole, D.E. & Quamme, G.A. Inherited disorders of renal magnesium handling. J. Am. Soc. Nephrol. 11, 1937–1947 (2000).
Walder, R.Y. et al. Hypomagnesemia with secondary hypocalcemia (HSH): narrowing the disease region on chromosome 9. Am. J. Hum. Genet. 65, 451 (1999).
Harteneck, C., Plant, T.D. & Schultz, G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 23, 159–166 (2000).
Shapiro, M.B. & Senapathy, P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 15, 7155–7174 (1987).
Quamme, G.A. Renal magnesium handling: new insights in understanding old problems. Kidney Int. 52, 1180–1195 (1997).
Fine, K.D., Santa Ana, C.A., Porter, J.L. & Fordtran, J.S. Intestinal absorption of magnesium from food and supplements. J. Clin. Invest. 88, 396–402 (1991).
Kayne, L.H. & Lee, D.B. Intestinal magnesium absorption. Miner. Electrolyte Metab. 19, 210–217 (1993).
Walder, R.Y. et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nature Genet. 31 (2002); advance online publication, 28 May 2002 (DOI:10.1038/ng901).
Simon, D.B. et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285, 103–106 (1999).
Meij, I.C. et al. Dominant isolated renal magnesium loss is caused by misrouting of the Na(+),K(+)-ATPase γ-subunit. Nature Genet. 26, 265–266 (2000).
Duncan, L.M. et al. Melastatin expression and prognosis in cutaneous malignant melanoma. J. Clin. Oncol. 19, 568–576 (2001).
Perraud, A.L. et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411, 595–599 (2001).
Cahalan, M.D. Cell biology. Channels as enzymes. Nature 411, 542–543 (2001).
Challa, A., Papaefstathiou, I., Lapatsanis, D. & Tsolas, O. Primary idiopathic hypomagnesemia in two female siblings. Acta Paediatr. 84, 1075–1078 (1995).
Konrad, M. et al. Mutations in the chloride channel gene CLCNKB as a cause of classic Bartter syndrome. J. Am. Soc. Nephrol. 11, 1449–1459 (2000).
Andre, E. et al. Disruption of retinoid-related orphan receptor β changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. EMBO J. 17, 3867–3877 (1998).
Reddy, S. et al. Isolation and characterization of a cDNA clone encoding a novel peptide (OSF) that enhances osteoclast formation and bone resorption. J. Cell Physiol. 177, 636–645 (1998).
Koizumi, K. et al. Cloning and expression of uridine/cytidine kinase cDNA from human fibrosarcoma cells. Int. J. Mol. Med. 8, 273–278 (2001).
Weber, S. et al. Primary gene structure and expression studies of rodent paracellin-1. J. Am. Soc. Nephrol. 12, 2664–2672 (2001).
Klingel, K. et al. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc. Natl Acad. Sci. USA 89, 314–318 (1992).
Acknowledgements
We thank the patients and their families for participating in this study, U. Pechmann and P. Barth for excellent technical assistance, C. Antignac, R. Preisig-Müller, C. Derst and N. Jeck for helpful discussions and C. Loirat, D. Lotan, W. Scheurlen, A. Siamopoulou, S. Alfandaki, G. Celsi and A. Kernell for providing clinical data. S.W., H.W.S. and M.K. were supported by the Deutsche Forschungsgemeinschaft. S.W. was supported by the Kempkes-Stiftung, University of Marburg. L.N.N. and S.N. were supported by the Danish National Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Schlingmann, K., Weber, S., Peters, M. et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31, 166–170 (2002). https://doi.org/10.1038/ng889
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng889
This article is cited by
-
TRPM channels in health and disease
Nature Reviews Nephrology (2024)
-
Lectin-mediated, time-efficient, and high-yield sorting of different morphologically intact nephron segments
Pflügers Archiv - European Journal of Physiology (2024)
-
Genetische Diagnostik der Epilepsien: Empfehlung der Kommission Epilepsie und Genetik der Deutschen Gesellschaft für Epileptologie (DGfE)
Clinical Epileptology (2023)
-
Identification of selection signatures in Iranian dromedary and Bactrian camels using whole genome sequencing data
Scientific Reports (2022)
-
Evidence for the expression of TRPM6 and TRPM7 in cardiomyocytes from all four chamber walls of the human heart
Scientific Reports (2021)