Abstract
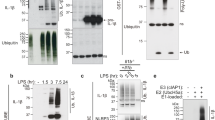
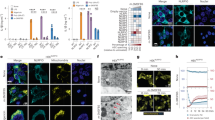
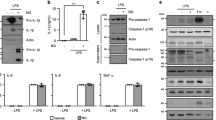
Autophagosomes delivers cytoplasmic constituents to lysosomes for degradation, whereas inflammasomes are molecular platforms activated by infection or stress that regulate the activity of caspase-1 and the maturation of interleukin 1β (IL-1β) and IL-18. Here we show that the induction of AIM2 or NLRP3 inflammasomes in macrophages triggered activation of the G protein RalB and autophagosome formation. The induction of autophagy did not depend on the adaptor ASC or capase-1 but was dependent on the presence of the inflammasome sensor. Blocking autophagy potentiated inflammasome activity, whereas stimulating autophagy limited it. Assembled inflammasomes underwent ubiquitination and recruited the autophagic adaptor p62, which assisted their delivery to autophagosomes. Our data indicate that autophagy accompanies inflammasome activation to temper inflammation by eliminating active inflammasomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Behrends, C., Sowa, M.E., Gygi, S.P. & Harper, J.W. Network organization of the human autophagy system. Nature 466, 68–76 (2010).
Schmid, D., Pypaert, M. & Munz, C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity 26, 79–92 (2007).
Deretic, V. Autophagy in innate and adaptive immunity. Trends Immunol. 26, 523–528 (2005).
Levine, B. & Deretic, V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat. Rev. Immunol. 7, 767–777 (2007).
Schmid, D. & Munz, C. Innate and adaptive immunity through autophagy. Immunity 27, 11–21 (2007).
Bodemann, B.O. et al. RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell 144, 253–267 (2011).
Xu, Y. et al. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity 27, 135–144 (2007).
Shi, C.S. & Kehrl, J.H. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J. Biol. Chem. 283, 33175–33182 (2008).
Delgado, M.A., Elmaoued, R.A., Davis, A.S., Kyei, G. & Deretic, V. Toll-like receptors control autophagy. EMBO J. 27, 1110–1121 (2008).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Trinchieri, G. & Sher, A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 7, 179–190 (2007).
Seibenhener, M.L. et al. Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol. Cell. Biol. 24, 8055–8068 (2004).
Pankiv, S. et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282, 24131–24145 (2007).
Ponpuak, M. et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity 32, 329–341 (2010).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Fernandes-Alnemri, T., Yu, J.W., Datta, P., Wu, J. & Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Bürckstümmer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272 (2009).
Roberts, T.L. et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060 (2009).
Lamkanfi, M. & Dixit, V.M. The inflammasomes. PLoS Pathog. 5, e1000510 (2009).
Rathinam, V.A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Fernandes-Alnemri, T. et al. The AIM2 inflammasome is critical for innate immunity to. Francisella tularensis Nat. Immunol. 11, 385–393 (2010).
Saitoh, T. et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature 456, 264–268 (2008).
Harris, J., Hope, J.C. & Lavelle, E.C. Autophagy and the immune response to TB. Transbound. Emerg. Dis. 56, 248–254 (2009).
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000).
Shi, C.S. & Kehrl, J.H. TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci. Signal. 3, ra42 (2010).
Mizushima, N., Yoshimori, T. & Levine, B. Methods in mammalian autophagy research. Cell 140, 313–326 (2010).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Sutterwala, F.S. et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 (2006).
Yamamoto, M. et al. ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells 9, 1055–1067 (2004).
Li, H. et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80, 401–411 (1998).
Yang, Z. & Klionsky, D.J. Mammalian autophagy: core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 22, 124–131 (2010).
Gutierrez, M.G. et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119, 753–766 (2004).
Master, S.S. et al. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe 3, 224–232 (2008).
Suzuki, T. et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 3, e111 (2007).
Dupont, N. et al. Shigella phagocytic vacuolar membrane remnants participate in the cellular response to pathogen invasion and are regulated by autophagy. Cell Host Microbe 6, 137–149 (2009).
Tschopp, J. & Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 (2010).
Zhou, R., Yazdi, A.S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Sanz, L., Diaz-Meco, M.T., Nakano, H. & Moscat, J. The atypical PKC-interacting protein p62 channels NF-κB activation by the IL-1-TRAF6 pathway. EMBO J. 19, 1576–1586 (2000).
Wooten, M.W. et al. The p62 scaffold regulates nerve growth factor-induced NF-kappaB activation by influencing TRAF6 polyubiquitination. J. Biol. Chem. 280, 35625–35629 (2005).
Ye, H. et al. Distinct molecular mechanism for initiating TRAF6 signalling. Nature 418, 443–447 (2002).
Bortoluci, K.R. & Medzhitov, R. Control of infection by pyroptosis and autophagy: role of TLR and NLR. Cell. Mol. Life Sci. 67, 1643–1651 (2010).
Kuida, K. et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science 267, 2000–2003 (1998).
Wertz, I.E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signaling. Nature 430, 694–699 (2004).
Fernandes-Alnemri, T. & Alnemri, E.S. Assembly, purification, and assay of the activity of the ASC pyroptosome. Methods Enzymol. 442, 251–270 (2008).
Gao, W. et al. Biochemical isolation and characterization of the tubulovesicular LC3-positive autophagosomal compartment. J. Biol. Chem. 285, 1371–1383 (2010).
Acknowledgements
We thank N. Mizushima (Tokyo Medical and Dental University) for LC3 cDNA; M. Rust for editorial assistance; and A. Fauci for support. Supported by the Intramural Research Program of the US National Institutes of Health (National Institute of Allergy and Infectious Diseases).
Author information
Authors and Affiliations
Contributions
C.-S.S. designed and did most of the experiments and helped write the manuscript; K.S. provided mouse macrophages and did the M. tuberculosis experiments; N.-N.H. did some of the confocal microscopy; J.K. analyzed images; M.A.-A. did the electron microscopy; K.A.F. provided the Aim2−/− cells and advice; A.S. helped in the design of several experiments and provided advice; and J.H.K. oversaw the experimental design, helped interpret the results and helped write the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 622 kb)
Supplementary Video 1
NLPR3 inflammasomes co-localize with GFP-LC3. (MOV 10851 kb)
Supplementary Video 2
ASC and p62 co-localization. (MOV 10769 kb)
Rights and permissions
About this article
Cite this article
Shi, CS., Shenderov, K., Huang, NN. et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol 13, 255–263 (2012). https://doi.org/10.1038/ni.2215
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2215
This article is cited by
-
Autophagy dysfunction contributes to NLRP1 inflammasome-linked depressive-like behaviors in mice
Journal of Neuroinflammation (2024)
-
The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases
Nature Reviews Cardiology (2024)
-
Selenomethionine Inhibits NF-κB-mediated Inflammatory Responses of Bovine Mammary Epithelial Cells Caused by Klebsiella pneumoniae by Increasing Autophagic Flux
Biological Trace Element Research (2024)
-
Resveratrol and quercetin protect from Benzo(a)pyrene-induced autophagy in retinal pigment epithelial cells
International Ophthalmology (2024)
-
FGF-18 Protects the Injured Spinal cord in mice by Suppressing Pyroptosis and Promoting Autophagy via the AKT-mTOR-TRPML1 axis
Molecular Neurobiology (2024)