Abstract
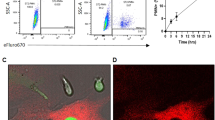
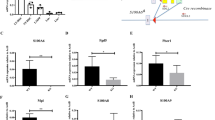
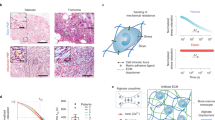
Hematopoietic stem and progenitor cells (HSPCs) are regulated by various bone marrow stromal cell types. Here we identified rare activated bone marrow monocytes and macrophages with high expression of α-smooth muscle actin (α-SMA) and the cyclooxygenase COX-2 that were adjacent to primitive HSPCs. These myeloid cells resisted radiation-induced cell death and further upregulated COX-2 expression under stress conditions. COX-2-derived prostaglandin E2 (PGE2) prevented HSPC exhaustion by limiting the production of reactive oxygen species (ROS) via inhibition of the kinase Akt and higher stromal-cell expression of the chemokine CXCL12, which is essential for stem-cell quiescence. Our study identifies a previously unknown subset of α-SMA+ activated monocytes and macrophages that maintain HSPCs and protect them from exhaustion during alarm situations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zhang, J. et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 (2003).
Calvi, L.M. et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 (2003).
Arai, F. et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161 (2004).
Sugiyama, T., Kohara, H., Noda, M. & Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988 (2006).
Sacchetti, B. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 (2007).
Spiegel, A., Kalinkovich, A., Shivtiel, S., Kollet, O. & Lapidot, T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell 3, 484–492 (2008).
Mendez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010).
Omatsu, Y. et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33, 387–399 (2010).
Yamazaki, S. et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146–1158 (2011).
Ding, L., Saunders, T.L., Enikolopov, G. & Morrison, S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Kiel, M.J. et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Fujisaki, J. et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 474, 216–219 (2011).
Chow, A. et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 208, 261–271 (2010).
Christopher, M.J., Rao, M., Liu, F., Woloszynek, J.R. & Link, D.C. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J. Exp. Med. 208, 251–260 (2010).
Winkler, I.G. et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 116, 4815–4828 (2010).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Crofford, L.J. COX-1 and COX-2 tissue expression: implications and predictions. J. Rheumatol. 24 (suppl. 49), 15–19 (1997).
Riese, J. et al. Transient expression of prostaglandin endoperoxide synthase-2 during mouse macrophage activation. J. Leukoc. Biol. 55, 476–482 (1994).
Lorenz, M. et al. Cyclooxygenase-2 is essential for normal recovery from 5-fluorouracil-induced myelotoxicity in mice. Exp. Hematol. 27, 1494–1502 (1999).
Hoggatt, J., Singh, P., Sampath, J. & Pelus, L.M. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood 113, 5444–5455 (2009).
Goessling, W. et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136, 1136–1147 (2009).
North, T.E. et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007–1011 (2007).
Gentile, P.S. & Pelus, L.M. In vivo modulation of myelopoiesis by prostaglandin E2. IV. Prostaglandin E2 induction of myelopoietic inhibitory activity. J. Immunol. 141, 2714–2720 (1988).
Pelus, L.M. Modulation of myelopoiesis by prostaglandin E2: demonstration of a novel mechanism of action in vivo. Immunol. Res. 8, 176–184 (1989).
Ito, K. et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 12, 446–451 (2006).
Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431, 997–1002 (2004).
Tothova, Z. et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 (2007).
Jang, Y.Y. & Sharkis, S.J. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110, 3056–3063 (2007).
Tesio, M. et al. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood 117, 419–428 (2011).
Akashi, K., Traver, D., Miyamoto, T. & Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000).
Magness, S.T., Bataller, R., Yang, L. & Brenner, D.A. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology 40, 1151–1159 (2004).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Farina, C., Theil, D., Semlinger, B., Hohlfeld, R. & Meinl, E. Distinct responses of monocytes to Toll-like receptor ligands and inflammatory cytokines. Int. Immunol. 16, 799–809 (2004).
Shi, C. et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating Toll-like receptor ligands. Immunity 34, 590–601 (2011).
Osawa, M., Hanada, K., Hamada, H. & Nakauchi, H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273, 242–245 (1996).
Kirouac, D.C. & Zandstra, P.W. Understanding cellular networks to improve hematopoietic stem cell expansion cultures. Curr. Opin. Biotechnol. 17, 538–547 (2006).
Yu, Y. et al. Genetic model of selective COX2 inhibition reveals novel heterodimer signaling. Nat. Med. 12, 699–704 (2006).
Juntilla, M.M. et al. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 115, 4030–4038 (2010).
Lewandowski, D. et al. In vivo cellular imaging pinpoints the role of reactive oxygen species in the early steps of adult hematopoietic reconstitution. Blood 115, 443–452 (2010).
Schajnovitz, A. et al. CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat. Immunol. 12, 391–398 (2011).
Dar, A., Kollet, O. & Lapidot, T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp. Hematol. 34, 967–975 (2006).
Goichberg, P. et al. cAMP-induced PKCζ activation increases functional CXCR4 expression on human CD34+ hematopoietic progenitors. Blood 107, 870–879 (2006).
Goessling, W. et al. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell 8, 445–458 (2011).
Yokota, T. et al. Bone marrow lacks a transplantable progenitor for smooth muscle type alpha-actin-expressing cells. Stem Cells 24, 13–22 (2006).
Huang, Y.L. et al. Thrombin induces nestin expression via the transactivation of EGFR signalings in rat vascular smooth muscle cells. Cell. Signal. 21, 954–968 (2009).
Gottlob, K. et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 15, 1406–1418 (2001).
Porter, R.L. & Calvi, L.M. in 52nd Annual Meeting of the American Society of Hematology Vol. 116, 181–181 (Blood, Orlando, Florida, 2010).
Hoggatt, J. et al. in 52nd Annual Meeting of the American Society of Hematology Vol. 116, 1088–1088 (Blood, Orlando, FL, 2010).
Golan, K. et al. S1P promotes murine progenitor cell egress and mobilization via S1P1 mediated ROS signaling and SDF-1 release. Blood 119, 2478–2488 (2012).
Petit, I. et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 3, 687–694 (2002).
Acknowledgements
We thank G.N. Enikolopov (Cold Spring Harbor Laboratory) for nestin-GFP mice; Y. Yarden (Weizmann Institute of Science) for the 3T3 cell line; N. Iscove for guidance during the study and for critical review of the manuscript; H. Perlman for help in the characterization of myeloid cells; J. Hanna for use of the FACSAria; and D. Shafritz, J. Canaani, K. Lapid and Y. Ovadia for critical review of the manuscript. Supported by the Israeli Science Foundation (ISF 544/09), the European Commission (CELL-PID FP7-261387) and the Leona M. and Harry B. Helmsley Charitable Trust.
Author information
Authors and Affiliations
Contributions
A.L. designed and did experiments, analyzed data and wrote the manuscript; T.I. and S.G.-C. helped in the design and execution of experiments and analyzed data; A.M. identified the myeloid characteristics of the α-SMA+ population; E.S. helped with the immunohistochemical staining; K.G., A.K., G.D. and A.S. helped with experiments; O.K. helped in writing the manuscript; Z.P. helped in the design and analysis of the flow cytometry–based single-cell imaging; E.V. designed and did IL-1β-related experiments; R.N.A. helped in the design of IL-1β-related experiments; D.A.B. provided α-SMA–RFP mice; S.J. provided advice and guided characterization of the α-SMA+ cells; and T.L. designed the research and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1794 kb)
Supplementary Video 1
Three dimensional view of α-SMA+ two cell complex adjacent to Nestin+ reticular cell. (AVI 122 kb)
Rights and permissions
About this article
Cite this article
Ludin, A., Itkin, T., Gur-Cohen, S. et al. Monocytes-macrophages that express α-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol 13, 1072–1082 (2012). https://doi.org/10.1038/ni.2408
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2408
This article is cited by
-
Cellular niches for hematopoietic stem cells in bone marrow under normal and malignant conditions
Inflammation and Regeneration (2023)
-
A specialized bone marrow microenvironment for fetal haematopoiesis
Nature Communications (2022)
-
M2 macrophages, but not M1 macrophages, support megakaryopoiesis by upregulating PI3K-AKT pathway activity
Signal Transduction and Targeted Therapy (2021)
-
A connexin/ifi30 pathway bridges HSCs with their niche to dampen oxidative stress
Nature Communications (2021)
-
Resistance of bone marrow stroma to genotoxic preconditioning is determined by p53
Cell Death & Disease (2021)