Abstract
Immunological memory is thought to be mediated exclusively by lymphocytes. However, enhanced innate immune responses caused by a previous infection increase protection against reinfection, which suggests the presence of innate immunological memory. Here we identified an important role for the stress-response transcription factor ATF7 in innate immunological memory. ATF7 suppressed a group of genes encoding factors involved in innate immunity in macrophages by recruiting the histone H3K9 dimethyltransferase G9a. Treatment with lipopolysaccharide, which mimics bacterial infection, induced phosphorylation of ATF7 via the kinase p38, which led to the release of ATF7 from chromatin and a decrease in repressive histone H3K9me2 marks. A partially disrupted chromatin structure and increased basal expression of target genes were maintained for long periods, which enhanced resistance to pathogens. ATF7 might therefore be important in controlling memory in cells of the innate immune system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ahmed, R., Bevan, M.J., Reiner, S.L. & Fearon, D.T. The precursors of memory: models and controversies. Nat. Rev. Immunol. 9, 662–668 (2009).
Kurtz, J. Specific memory within innate immune systems. Trends Immunol. 26, 186–192 (2005).
Durrant, W.E. & Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 42, 185–209 (2004).
Rodrigues, J., Brayner, F.A., Alves, L.C., Dixit, R. & Barillas-Mury, C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science 329, 1353–1355 (2010).
Pham, L.N., Dionne, M.S., Shirasu-Hiza, M. & Schneider, D.S. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 3, e26 (2007).
Quintin, J. et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12, 223–232 (2012).
Gorhe, D.S. Inhibition of multiplication of foot and mouth disease virus in adult mice pretreated with Freund's complete adjuvant. Nature 216, 1242–1244 (1967).
Kleinnijenhuis, J. et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 109, 17537–17542 (2012).
Barton, E.S. et al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447, 326–329 (2007).
Netea, M.G., Quintin, J. & van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 9, 355–361 (2011).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014).
Cheng, S.C. et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684 (2014).
Gaire, M., Chatton, B. & Kedinger, C. Isolation and characterization of two novel, closely related ATF cDNA clones from HeLa cells. Nucleic Acids Res. 18, 3467–3473 (1990).
Maekawa, T. et al. Leucine zipper structure of the protein CRE-BP1 binding to the cyclic AMP response element in brain. EMBO J. 8, 2023–2028 (1989).
Hai, T.W., Liu, F., Coukos, W.J. & Green, M.R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 3, 2083–2090 (1989).
Seong, K.H., Li, D., Shimizu, H., Nakamura, R. & Ishii, S. Inheritance of stress-induced, ATF-2-dependent epigenetic change. Cell 145, 1049–1061 (2011).
Maekawa, T. et al. Social isolation stress induces ATF-7 phosphorylation and impairs silencing of the 5-HT 5B receptor gene. EMBO J. 29, 196–208 (2010).
Gupta, S., Campbell, D., Dérijard, B. & Davis, R.J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science 267, 389–393 (1995).
Seong, K.H., Maekawa, T. & Ishii, S. Inheritance and memory of stress-induced epigenome change: roles played by the ATF-2 family of transcription factors. Genes Cells 17, 249–263 (2012).
Hirose, N., Maekawa, T., Shinagawa, T. & Ishii, S. ATF-2 regulates lipopolysaccharide-induced transcription in macrophage cells. Biochem. Biophys. Res. Commun. 385, 72–77 (2009).
Shivers, R.P. et al. Phosphorylation of the conserved transcription factor ATF-7 by PMK-1 p38 MAPK regulates innate immunity in Caenorhabditis elegans. PLoS Genet. 6, e1000892 (2010).
Ghisletti, S. et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity 32, 317–328 (2010).
Shinkai, Y. & Tachibana, M. H3K9 methyltransferase G9a and the related molecule GLP. Genes Dev. 25, 781–788 (2011).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Ahmad, K. & Henikoff, S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell 9, 1191–1200 (2002).
Tachibana, M. et al. Histone methyltransferases G9a and GLP form heteromeric complexes and are both crucial for methylation of euchromatin at H3–K9. Genes Dev. 19, 815–826 (2005).
Biswas, S.K. & Lopez-Collazo, E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 (2009).
Hiroi, M. & Ohmori, Y. The transcriptional coactivator CREB-binding protein cooperates with STAT1 and NF-κB for synergistic transcriptional activation of the CXC ligand 9/monokine induced by interferon-gamma gene. J. Biol. Chem. 278, 651–660 (2003).
Groom, J.R. & Luster, A.D. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 89, 207–215 (2011).
Ichimura, T. et al. Transcriptional repression and heterochromatin formation by MBD1 and MCAF/AM family proteins. J. Biol. Chem. 280, 13928–13935 (2005).
Foster, S.L., Hargreaves, D.C. & Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447, 972–978 (2007).
Kim, J. et al. RAD6-Mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell 137, 459–471 (2009).
van Essen, D. et al. A feed-forward circuit controlling inducible NF-κB target gene activation by promoter histone demethylation. Mol. Cell 39, 750–760 (2010).
Medzhitov, R. & Horng, T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 9, 692–703 (2009).
Davies, L.C., Jenkins, S.J., Allen, J.E. & Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 14, 986–995 (2013).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Hashimoto, D. et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 (2013).
Davies, L.C. et al. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat. Commun. 4, 1886 (2013).
Ghosn, E.E. et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc. Natl. Acad. Sci. USA 107, 2568–2573 (2010).
Sun, J.C., Beilke, J.N. & Lanier, L.L. Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009).
Benn, C.S., Netea, M.G., Selin, L.K. & Aaby, P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 34, 431–439 (2013).
Yazdanbakhsh, M., Kremsner, P.G. & van Ree, R. Allergy, parasites, and the hygiene hypothesis. Science 296, 490–494 (2002).
Luster, A.D. & Ravetch, J.V. Biochemical characterization of a γ-interferon-inducible cytokine (IP-10). J. Exp. Med. 166, 1084–1097 (1987).
Farber, J.M. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc. Natl. Acad. Sci. USA 87, 5238–5242 (1990).
Ishibashi, K. et al. Relationship between the physical properties of Candida albicans cell well β-glucan and activation of leukocytes in vitro. Int. Immunopharmacol. 2, 1109–1122 (2002).
Wendt, K.S. et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 451, 796–801 (2008).
Liu, C.L. et al. Development and validation of a T7 based linear amplification for genomic DNA. BMC Genomics 4, 19 (2003).
Johnson, W.E. et al. Model-based analysis of tiling-arrays for ChIP-chip. Proc. Natl. Acad. Sci. USA 103, 12457–12462 (2006).
Droit, A. et al. rMAT–an R/Bioconductor package for analyzing ChIP-chip experiments. Bioinformatics 26, 678–679 (2010).
Frith, M.C. et al. Detection of functional DNA motifs via statistical over-representation. Nucleic Acids Res. 32, 1372–1381 (2004).
Bryne, J.C. et al. JASPAR, the open access database of transcription factor-binding profiles: new content and tools in the 2008 update. Nucleic Acids Res. 36, D102–D106 (2008).
Badis, G. et al. Diversity and complexity in DNA recognition by transcription factors. Science 324, 1720–1723 (2009).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc., B 57, 289–300 (1995).
Smyth, G.K. (2005). Limma: linear models for microarray data. In Bioinformatics and Computational Biology Solutions using R and Bioconductor (eds. Gentleman, R., Carey, V., Dudoit, S., Irizarry, R. & Hube, W.) 397–420 (Springer, 2005).
Sano, Y. et al. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-β signaling. J. Biol. Chem. 274, 8949–8957 (1999).
Krämer, A., Green, J., Pollard, J. Jr. & Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530 (2014).
Xue, J. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288 (2014).
Acknowledgements
We thank K. Morikawa (University of Tsukuba) for S. aureus; the staff of the Research Resources Center of the RIKEN Brain Science Institute for microarray analysis; and Y. Shinkai (RIKEN) for discussions. Supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science, and Technology.
Author information
Authors and Affiliations
Contributions
K.Y. performed most of the experiments; Y.Z. performed immunoblot analysis and ChIP experiments; T.M. supported the mouse experiments; C.R.-G. and K.S. analyzed the data from gene-expression profiling and ChIP-chip experiments; B.C. supplied monoclonal antibodies; K. Inoue., T.U., T.Y. and M.O.-H. analyzed the network; K.-i. Ishibashi and N.O. supplied C. albicans BG; S.I. conceived of and supervised the study; and S.I. and K.Y. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Morphology of wild-type and Atf7−/− macrophages, and binding of ATF7 at loci identified by ChIP-chip assays.
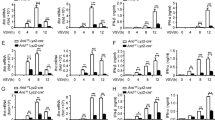
(a) Morphological differences between WT and Atf7−/− macrophages. LPS-treated or non-treated peritoneal macrophages from wild-type (WT) and Atf7−/− mice were stained with anti–α-tubulin antibody (green), and DNA was stained with TO-PRO-3 (blue). Scale bar: 20 μm. Typical cells present in the white boxes are shown below at higher magnification. Data are representative of three experiments using three mice of each group. (b) Purity of peritoneal macrophages. Prepared macrophages were stained with anti-CD68 (green), and DNA was stained with TO-PRO-3 (blue). Images were obtained by confocal microscopy. Note that most of the cells are CD68-positive. Scale bar: 50 μm. Data are representative of three experiments using three mice of each group. (c) ATF7 ChIP-chip data at typical innate-immunity genes. MAT (Model-based Analysis of Tiling-arrays) score obtained from three experiments using three mice of each group is shown. (d) Quantitative ChIP assays were performed using peritoneal macrophages and anti-ATF7 antibody. Primers covering 26 ATF7 binding sites that were identified by ChIP-chip assays, while primers covering five sites that did not bind ATF7 were used as negative controls. The amounts of amplified DNA relative to the input DNA were determined, and are shown as means ± SD (n = 3 mice for each group).
Supplementary Figure 2 Identification of ATF7 domains that interact with G9a.
(a) Whole-cell lysates from the macrophage cell line J774.1 were immunoprecipitated with anti-G9a antibody or control IgG, and the immunocomplexes were subjected to Western blotting with anti-ATF7 or anti-G9a antibody. (b) GST-ATF7 containing various forms of ATF7. (c) GST precipitation assay was performed using nuclear extracts from cell line J774.1 and the constructs shown in (b). The binding activities of various forms of ATF7 are shown as + and – on the right. GST-ATF7 fusions were purified using glutathione-Sepharose beads and then analyzed by SDS-PAGE followed by Coomassie blue staining. Full-length forms are indicated by arrows. (d) After mixing macrophage extracts and glutathione-Sepharose beads to which GST-ATF7 was bound, resins were washed, and bound G9a was analyzed by SDS-PAGE followed by Western blotting. Two forms of G9a generated by alternative splicing were detected. All of the data (a, c, and d) are representative of two experiments.
Supplementary Figure 3 Phosphorylation of ATF7 in response to LPS treatment, followed by its release from target genes.
(a) Time course of ATF7 phosphorylation by LPS. Cells of the mouse macrophage line J774.1 were treated with LPS (100 ng/ml), and whole-cell lysates were prepared at the indicated times. Lysates were used for Western blotting with anti-pATF2/7 and anti-ATF7. ATF2 and ATF7 are separated by SDS-PAGE, and only pATF7 is shown. (Lower) SB239063, a p38 inhibitor, was added 1 h before addition of LPS, and ATF7 and pATF7 were analyzed as described above. (b) Mice were injected with LPS (5 μg), and peritoneal macrophages were prepared at the indicated times after LPS injection. Whole-cell lysates were prepared and then subjected to Western blotting using anti-pATF2/7 or anti-ATF7 antibodies. ATF2 and ATF7 were separated by SDS-PAGE, and only pATF7 is shown. (c) Subcellular localization of phosphorylated p38 (pp38) and pATF7 in J774.1 cells. At the indicated times after the addition of LPS (100 ng/ml), whole cell lysates, cytosol and nuclear fractions were prepared and used for Western blotting to detect ATF7, pATF7, p38, or pp38. α-Tubulin and histone H3 were used as the control. (d) Subcellular localization of pATF7 in human monocyte cell line THP-1 after differentiation into macrophage by PMA treatment. At the indicated times after the addition of LPS (100 ng/ml), cytosol and nuclear fractions were prepared and used for Western blotting to detect ATF7 or pATF7. All of Western blotting data (a, b, c, and d) are representative of two experiments. (e) Release of ATF7 from the target genes upon LPS treatment. J774.1 cells were treated with LPS (100 ng/ml) for 7 h or non-treated, and then subjected to ChIP assays using anti-ATF7 antibody. The amounts of amplified DNA relative to the input DNA were determined and are shown as means ± SD (n = 3 mice for each group). * P < 0.05 and ** P < 0.01 (unpaired Student’s t-test). Note that amounts of ATF7 on target genes in J774.1 cells are lower than those in primary macrophages (compare the data in Fig. 4b). Given that culture of J774.1 cells in serum-free medium increases the amount of ATF7 on target genes, the lower amount of ATF7 in J774.1 cells might be due to the effect of residual inflammatory cytokines, including TNF, in serum.
Supplementary Figure 4 Gene expression, ATF7 release, and histone-modification change at the acute induction phase after LPS treatment.
(a) Induction of expression of ATF7 target genes by LPS. Peritoneal macrophages from WT and Atf7−/− mice were treated with LPS (100 ng/ml), and RNA was prepared at the indicated times. RT-qPCR was performed to examine the mRNA levels corresponding to the indicated genes. Values relative to levels in cells not treated with LPS are shown as means + SD (n = 3 mice for each group). (b) Effect of LPS on H3 and H3K9me2 localization on the Magea2 gene, which binds G9a but not ATF7. WT resident macrophages were treated with 100 ng/ml LPS for the indicated times, and then the Magea2 promoter region was subjected to ChIP assays using anti-H3K9me2, anti-H3, or anti-H3.3 antibody. Values relative to levels in cells not treated with LPS treatment are shown as means + SD (n = 2 mice for each sample). (c) WT or Atf7−/− macrophages were treated with 100 ng/ml of LPS or PBS for 12 h and then subjected to ChIP assays with anti-H3K9me2 and anti-H3 antibodies. Values relative to levels in WT cells treated with PBS are shown as means + SD (n = 3 mice for each group). NS, not significant. (d) LPS releases ATF7 from some innate immunity-related genes in human monocyte cell line THP-1. THP-1 cells were treated with LPS (100 ng/ml) or PBS (control) for 7 h, and then subjected to ChIP assays using anti-ATF7 antibody or IgG (mock). Extracted DNA was amplified by real-time PCR using primers that cover the ATF7 binding site on each gene’s promoter region. The amount of amplified DNA relative to the input DNA was determined, and is shown as means + SD (n = 3 mice for each group). (e) ChIP assays were carried out on THP-1 cells using anti-histone H3K9me2 and anti-H3 antibodies 7 h after LPS (100 ng/ml) treatment, and the ratio of H3K9me3 to H3 is shown. Each bar represents the mean ± SD (n = 3 mice for each group). *P < 0.05, **P < 0.01, and ***P < 0.001 (unpaired Student’s t-test or Welch’s t-test).
Supplementary Figure 5 Gene expression, ATF7 release, and histone-modification change at the tolerance phase after LPS treatment.
(a) Tolerance at 3 days after LPS treatment. WT or Atf7−/− mice were injected with LPS (5 μg) or PBS, and 3 days later macrophages were prepared and re-challenged with LPS (100 ng/ml) and the levels of Cxcl2 and Tnf mRNA were measured by qRT-PCR. Expression levels relative to those in cells without LPS re-challenge are shown as means + SD (n = 3 mice for each group). (b) Maintenance of reduced levels of ATF7. Mice were injected with LPS (5 μg); 3 days later, macrophages were prepared and subjected to ChIP assays using anti-ATF7 antibody. The amounts of amplified DNA relative to the input DNA were determined, and values relative to levels in animals treated with PBS are shown as means + SD (n = 3 mice for each group). (c) Maintenance of reduced level of H3K9me2. Mice were injected with LPS; macrophages were prepared as described above and then used for ChIP assays with anti-H3K9m2 and anti-H3 antibodies. Ratios of H3K9me2 to H3, relative to the ratio in animals treated with PBS, are shown as means + SD (n = 3 mice for each group). (d) Lower basal expression level of ATF7 target genes. Mice were injected with LPS; macrophages were prepared as described above and then subjected to qRT-PCR. Expression levels of the indicated genes, relative to the levels in animals treated with PBS, are shown as means + SD (n = 3 mice for each group). NS, not significant; *P < 0.05 and **P < 0.01 (unpaired Student’s t-test or Welch’s t-test).
Supplementary Figure 6 Maintenance of higher basal expression of ATF7’s target genes at the resting state after LPS treatment.
(a) No tolerance was evident at 3 weeks after LPS injection. WT or Atf7−/− mice were injected with LPS (5 μg) or PBS, and 3 weeks later macrophages were prepared and re-challenged with LPS (100 ng/ml) and the levels of Cxcl2 and Tnf mRNA were measured by qRT-PCR. Expression levels relative to those in cells without LPS re-challenge are shown as means + SD (n = 3 mice for each group). NS, not significant. (b) Maintenance of increased basal expression level of ATF7 target genes at 3 weeks after LPS treatment. Mice were injected with the indicated amounts of LPS or PBS; 3 weeks later, macrophage RNA was isolated and subjected to qRT-PCR. Expression levels of the indicated genes, relative to the levels in animals treated with PBS, are shown as means + SD (n = 3 mice for each group). *, p < 0.05; **, p < 0.01. (c) Percent overlap between genes upregulated in Atf7−/− macrophages and genes upregulated in WT cells (p < 0.05, fold change > 1.3) as a function of the degree of induction 3 weeks after LPS treatment (x axis). (d) Induction level of CCL3 by low-concentration of LPS by re-challenge. WT mice were injected with LPS (5 μg), and 3 weeks later, macrophages were prepared and treated with indicated concentration of LPS for 12 h, and CCL3 level was measured using ELISA (n = 4 mice for each group). NS, not significant. (e) Maintenance of increased expression of activation markers at 3 weeks after LPS injection. WT and Atf7−/− mice were injected with LPS (5 μg) or PBS, and 3 weeks later macrophage activation marker MHCII on peritoneal macrophages was examined by flow cytometry. Shown are representative of three experiments using three mice of each group. Percentage of MHCII-positive cells is shown in the bar graph on the right. Values indicate the means + SD (n = 3 mice for each group). NS, not significant; *P < 0.05 and **P < 0.01 (unpaired Student’s t-test or Welch’s t-test).
Supplementary Figure 7 Gene expression at 3 months after LPS injection, and amount of histone H3.3 and HP1γ at 3 weeks after LPS injection.
(a) (b) Mice were injected with LPS (5 μg) or PBS; 3 weeks later, macrophages were isolated and used for ChIP-assays using anti-HP1γ (a) or anti-histone H3.3 (b). Levels relative to those in cells treated with PBS are shown as means + SD (n = 4 mice for each group). (c) Maintenance of increased basal expression level of some ATF7 target genes at 3 months after LPS treatment. Mice were injected with LPS (5 μg) or PBS; 3 months later, macrophage RNA was isolated and used for qRT-PCR. Expression levels of the indicated genes, relative to the levels in animals treated with PBS, are shown as means + SD (n = 4 mice for each group). NS, not significant; *P < 0.05 and **P < 0.01 (unpaired Student’s t-test or Welch’s t-test).
Supplementary Figure 8 Network analysis of genes upregulated in LPS-primed macrophages.
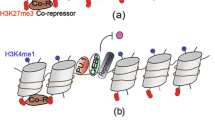
(a) Genes upregulated (fold change > 1.3) in mice treated with LPS relative to those treated with PBS were extracted, and Upstream Analysis was performed using the Ingenuity Pathway Analysis (IPA) software. The results predicted that STAT1 is a significantly activated transcription factor (activation z-score 5.385) associated with induction of mRNAs encoding STAT1 target genes. A predicted Mechanistic Network of STAT1 regulation is shown (orange and blue colors show predicted activation and down-regulation, respectively; solid and dotted lines show direct and indirect interactions, respectively). (b) Genes upregulated (fold change > 1.3) in Atf7−/− mice treated with LPS relative to those treated with PBS were extracted and subjected to Upstream Analysis using the Ingenuity Pathway Analysis (IPA) software (QIAGEN). The result predicted that STAT1 is a strongly activated transcription factor. A predicted Mechanistic Network of STAT1 regulation is shown (orange and blue show predicted activation and down-regulation, respectively; solid and dotted lines show direct and indirect interactions, respectively). STAT1 target genes are also shown (red is associated with mRNA induction). (c) WT and Atf7−/− mice were injected with LPS (5 μg) or PBS, and 3 weeks later RNA was prepared from resident macrophages, and used for qRT-PCR to measure indicated mRNA. Expression levels of the indicated genes, relative to the levels in WT animals treated with PBS, are shown as means + SD (n = 4 mice for each group). (d) Schematic representation of the memory of ATF7-dependent epigenetic changes induced by pathogen infection. ATF7 silences a group of innate-immunity genes by recruiting G9a, a histone H3K9 dimethyltransferase. Pathogen infection induces ATF7 phosphorylation via TLR and p38, resulting in a reduction in the levels of both ATF7 and H3K9me2. After transcriptional induction is turned off, transcription of those genes is temporarily inhibited during the tolerance stage by induction of signaling inhibitor(s), although the reduction in H3K9me2 level is maintained. After the tolerance stage, because ATF7 is not recruited back to these genes, H3K9me2 does not completely recover to the original level, and a slightly reduced H3K9me2 level and higher basal expression of target genes are sustained for long periods. Higher basal expression level of target genes contributes to resistance to pathogens.
Supplementary Figure 9 Network analysis of genes upregulated in BG-primed macrophages.
(a) Genes upregulated (fold change > 1.3) in mice treated with BG relative to those treated with PBS were extracted, and Upstream Analysis was performed using IPA. The results predicted that STAT1 is a significantly activated transcription factor (activation z-score 4.808) associated with induction of mRNAs encoding STAT1 target genes. The results are presented as described in Supplementary Fig. 8a. (b) Similarity for upregulated genes at 3 weeks in LPS- or BG-primed macrophases (LPS_3w or BG_3w) and genes after various stimulations in M1 and M2 macrophages. Clustering of Xue’s data59 and our data (LPS_3w or BG_3w) based on 42 upregulated genes (fold change > 1.3 and FDR < 0.05).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1 and 2 (PDF 1763 kb)
Rights and permissions
About this article
Cite this article
Yoshida, K., Maekawa, T., Zhu, Y. et al. The transcription factor ATF7 mediates lipopolysaccharide-induced epigenetic changes in macrophages involved in innate immunological memory. Nat Immunol 16, 1034–1043 (2015). https://doi.org/10.1038/ni.3257
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3257
This article is cited by
-
Processing stimulus dynamics by the NF-κB network in single cells
Experimental & Molecular Medicine (2023)
-
G9a/GLP inhibition during ex vivo lymphocyte expansion increases in vivo cytotoxicity of engineered T cells against hepatocellular carcinoma
Nature Communications (2023)
-
HP1a-mediated heterochromatin formation promotes antimicrobial responses against Pseudomonas aeruginosa infection
BMC Biology (2022)
-
Role of the Hematopoietic Stem Cells in Immunological Memory
Current Stem Cell Reports (2022)
-
Systemic administration of β-glucan induces immune training in microglia
Journal of Neuroinflammation (2021)