Abstract
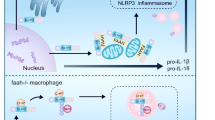
Inflammasomes are positioned to rapidly escalate the intensity of inflammation by activating interleukin (IL)-1β, IL-18 and cell death by pyroptosis. However, negative regulation of inflammasomes remains poorly understood, as is the signaling cascade that dampens inflammasome activity. We found that rapid NLRP3 inflammasome activation was directly inhibited by protein kinase A (PKA), which was induced by prostaglandin E2 (PGE2) signaling via the PGE2 receptor E-prostanoid 4 (EP4). PKA directly phosphorylated the cytoplasmic receptor NLRP3 and attenuated its ATPase function. We found that Ser295 in human NLRP3 was critical for rapid inhibition and PKA phosphorylation. Mutations in NLRP3-encoding residues adjacent to Ser295 have been linked to the inflammatory disease CAPS (cryopyrin-associated periodic syndromes). NLRP3-S295A phenocopied the human CAPS mutants. These data suggest that negative regulation at Ser295 is critical for restraining the NLRP3 inflammasome and identify a molecular basis for CAPS-associated NLRP3 mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Latz, E., Xiao, T.S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Masters, S.L., Simon, A., Aksentijevich, I. & Kastner, D.L. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*). Annu. Rev. Immunol. 27, 621–668 (2009).
Romberg, N. et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat. Genet. 46, 1135–1139 (2014).
Canna, S.W. et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat. Genet. 46, 1140–1146 (2014).
Strowig, T., Henao-Mejia, J., Elinav, E. & Flavell, R. Inflammasomes in health and disease. Nature 481, 278–286 (2012).
Taskén, K. & Aandahl, E.M. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol. Rev. 84, 137–167 (2004).
Yan, Y. et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73 (2015).
Lee, G.S. et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492, 123–127 (2012).
Sokolowska, M. et al. Prostaglandin E2 inhibits NLRP3 inflammasome activation through EP4 receptor and intracellular cyclic AMP in human macrophages. J. Immunol. 194, 5472–5487 (2015).
Serhan, C.N. & Savill, J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197 (2005).
Hirata, T. & Narumiya, S. Prostanoid receptors. Chem. Rev. 111, 6209–6230 (2011).
Kunkel, S.L., Chensue, S.W. & Phan, S.H. Prostaglandins as endogenous mediators of interleukin 1 production. J. Immunol. 136, 186–192 (1986).
Takaishi, O. et al. Inhibition by 16,16-dimethyl prostaglandin E2 of tumor necrosis factor-alpha and interleukin-1beta production and messenger RNA expression in human monocytes stimulated by Helicobacter pylori. Dig. Dis. Sci. 44, 2405–2411 (1999).
Kunkel, S.L. et al. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J. Biol. Chem. 263, 5380–5384 (1988).
Marotta, P., Sautebin, L. & Di Rosa, M. Modulation of the induction of nitric oxide synthase by eicosanoids in the murine macrophage cell line J774. Br. J. Pharmacol. 107, 640–641 (1992).
Takayama, K. et al. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J. Biol. Chem. 277, 44147–44154 (2002).
Fernandes-Alnemri, T. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 (2007).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Agostini, L. et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20, 319–325 (2004).
Gloerich, M. & Bos, J.L. Epac: defining a new mechanism for cAMP action. Annu. Rev. Pharmacol. Toxicol. 50, 355–375 (2010).
Rehmann, H., Schwede, F., Døskeland, S.O., Wittinghofer, A. & Bos, J.L. Ligand-mediated activation of the cAMP-responsive guanine nucleotide exchange factor Epac. J. Biol. Chem. 278, 38548–38556 (2003).
Dalton, G.D. & Dewey, W.L. Protein kinase inhibitor peptide (PKI): a family of endogenous neuropeptides that modulate neuronal cAMP-dependent protein kinase function. Neuropeptides 40, 23–34 (2006).
Aganna, E. et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 46, 2445–2452 (2002).
Dodé, C. et al. New mutations of CIAS1 that are responsible for Muckle-Wells syndrome and familial cold urticaria: a novel mutation underlies both syndromes. Am. J. Hum. Genet. 70, 1498–1506 (2002).
Aróstegui, J.I. et al. Clinical and genetic heterogeneity among Spanish patients with recurrent autoinflammatory syndromes associated with the CIAS1/PYPAF1/NALP3 gene. Arthritis Rheum. 50, 4045–4050 (2004).
Feldmann, J. et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 71, 198–203 (2002).
Neven, B. et al. Molecular basis of the spectral expression of CIAS1 mutations associated with phagocytic cell-mediated autoinflammatory disorders CINCA/NOMID, MWS, and FCU. Blood 103, 2809–2815 (2004).
Leinonen, M., Hallén, B. & Olivecrona, H. The IL-1 receptor antagonist anakinra stabilizes the NLRP3 mutation-specific risk for hearing loss in patients with severe cryopyrin-associated periodic syndromes (CAPS). Pediatr. Rheumatol. Online J. 12 (Suppl. 1), 76 (2014).
Kanariou, M. et al. Twenty year follow up of a patient with a new de-novo NLRP3 mutation (S595G) and CINCA syndrome. Klin. Padiatr. 221, 379–381 (2009).
Taylor, S.S. et al. Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design. Biochim. Biophys. Acta 1784, 16–26 (2008).
Duncan, J.A. et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc. Natl. Acad. Sci. USA 104, 8041–8046 (2007).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Ng, J. et al. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology 139, 542–552, 552.e1–552.e3 (2010).
Masters, S.L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 (2010).
Hentze, H., Lin, X.Y., Choi, M.S. & Porter, A.G. Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ. 10, 956–968 (2003).
Misawa, T. et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 14, 454–460 (2013).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Ippagunta, S.K. et al. The inflammasome adaptor ASC regulates the function of adaptive immune cells by controlling Dock2-mediated Rac activation and actin polymerization. Nat. Immunol. 12, 1010–1016 (2011).
Susuki-Miyata, S. et al. Cross-talk between PKA-Cβ and p65 mediates synergistic induction of PDE4B by roflumilast and NTHi. Proc. Natl. Acad. Sci. USA 112, E1800–E1809 (2015).
Myeku, N. et al. Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling. Nat. Med. 22, 46–53 (2016).
Schmitz, T. et al. Prostaglandin E2 receptor expression in fetal baboon lung at 0.7 gestation after betamethasone exposure. Pediatr. Res. 61, 421–426 (2007).
Dey, I., Giembycz, M.A. & Chadee, K. Prostaglandin E(2) couples through EP(4) prostanoid receptors to induce IL-8 production in human colonic epithelial cell lines. Br. J. Pharmacol. 156, 475–485 (2009).
Lejeune, M., Leung, P., Beck, P.L. & Chadee, K. Role of EP4 receptor and prostaglandin transporter in prostaglandin E2-induced alteration in colonic epithelial barrier integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1097–G1105 (2010).
Medina, D.L. et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 17, 288–299 (2015).
He, Y. et al. 3,4-methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J. Biol. Chem. 289, 1142–1150 (2014).
Acknowledgements
We thank Y. Shi (University of Calgary) for MSU and silica. Supported by the Canadian Institute of Health Research (MOP-340405 to K.C.; Tier 1 Canada Research Chair in Gastrointestinal Inflammation to K.C.; Team Grant in Health Challenges in Chronic Inflammation to J.A.M.), the Natural Sciences and Engineering Research Council of Canada (RGPIN-04023 to K.C.; and Canada Graduate Scholarship to L.M.) and Alberta Innovates Health Solutions (J.A.M. and L.M.).
Author information
Authors and Affiliations
Contributions
L.M., J.A.M. and K.C. conceived of and designed the experiments and wrote the paper; L.M., F.M. and J.A.M. performed the experiments and analyzed the data; and L.M., F.M., J.A.M. and K.C. contributed reagents, materials and analysis tools.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 PGE2 does not induce degradation of NLRP3 inflammasome components but rapidly inhibits NLRP3 activation in macrophages.
(a-d) LPS-primed BMDM. (a) Lysates (LYS) from PGE2 (500 nM) treatment for 30 min. (b) The indicated concentration of PGE2 was added for 5 min followed by stimulation with (left) nigericin (5 μM for 30 min) or (right) ATP (5 mM for 45 min). (c) PGE2 (500 nM) was added 30 min after stimulation with nigericin and analyzed 50 min post initial nigericin stimulation. (d) 16,16-dimethyl PGE2 (16-DM, 500 nM) or 15-keto PGE2 (15-keto, 500 nM) were added 5 min prior to nigericin or ATP stimulation. (e-g) PMA-differentiated THP-1 cells were treated with (e,f) the indicated concentration of PGE2 for 5 min prior to stimulation with (e) nigericin (5 μM) and LPS (50 ng/ml) for 30 min or (f) ATP (5 mM) and LPS (50 ng/ml) for 45 min. (g) 16,16-dimethyl PGE2 (16-DM, 250 nM), or 15-keto PGE2 (15-keto, 250 nM) were added 5 min prior to nigericin. (b-g) Total IL-1β release in cell supernatants analyzed by ELISA. *P < 0.005 (unpaired two-tailed t test). (a-g) Data are from one experiment representative of three separate experiments with similar results, (a-d) BMDM were derived from 2 mice per experiment mixed together, (b-g) three replicates, each replicate is one well + s.e.m.
Supplementary Figure 2 Particle-induced NLRP3 inflammasome activation is partially suppressed by PGE2, and cyclooxygenase inhibition blocks NLRP3 activation.
(a-c) BMDM were primed with LPS. (a,b) The indicated concentration of PGE2 was added 5 min before stimulation with (left) silica (250 μg/ml for 5 h) or (right) MSU (150 μg/ml for 4 h). (c) Indomethacin (50 μM) was added 30 min before stimulation with nigericin (5 μM for 30 min). (a,c) Total IL-1β release in cell supernatants analyzed by ELISA. (b,c) Immunoblot analysis of culture supernatants (SN) and lysates (LYS). *P < 0.005 (unpaired two-tailed t test). (a-c) Data are from one experiment representative of three separate experiments with similar results, (a-c) BMDM were derived from 2 mice per experiment mixed together, (a,c) three replicates, each replicate is one well + s.e.m.
Supplementary Figure 3 PGE2 receptors EP1 and EP3 do not inhibit, but EP4 does inhibit, the NLRP3 inflammasome.
(a-i) BMDMs primed for 3.5 h with LPS. (a,b) The indicated concentration of EP1 agonist ONO-DI-004 or (c,d) EP3 agonist ONO-AE-248 was added 5 min prior to (a,c) nigericin (5 μM for 30 min) or (b,d) ATP (5 mM for 45 min). (e-g) The indicated concentration of EP4 agonists (e left,f) CAY10598 and (e right,g) ONO-AE1-32 were added 5 min before (e) nigericin (5 μM for 30 min) or (f,g) ATP (5 mM for 45 min). (h,i) An EP4 antagonist ONO-AE3-208 (2 μM) was added for 5 min prior to addition of ONO-AE1-329 (500 nM) for 5 min and then treated with (h) nigericin (5 μM for 30 min) or (i) ATP (5 mM for 45 min). (a-i) Total IL-1β release in cell supernatants analyzed by ELISA. (a-d,f,g,i) Immunoblot analysis of culture supernatants (SN) and lysates (LYS). *P < 0.005 (unpaired two-tailed t test). (a-i) Data are from one experiment representative of three separate experiments with similar results, (a-i) BMDM were derived from 2 mice per experiment mixed together, (a-i) three replicates, each replicate is one well + s.e.m.
Supplementary Figure 4 PGE2 receptors EP2 and EP4 inhibit the NLRP3 inflammasome, but EP4 mediates PGE2-induced inhibition.
(a-h) BMDMs primed for 3.5 h with LPS. (a,b) The indicated concentration of EP2 agonist ONO-AE1-259-01 was added 5 min before treatment with (a) nigericin (5 μM for 30 min) or (b) ATP (5 mM 45 min). (c) The EP2 antagonist AH6809 (2 μM) was added 5 min prior to addition of ONO-AE1-259-01 followed by ATP (5 mM 45 min). (d) CAY10598 (500 nM) was added 30 min after stimulation with nigericin and analyzed 50 min post initial nigericin stimulation. (e,f) EP2 agonist ONO-AE1-259-01 or (g,h) EP4 antagonist ONO-AE3-208 was added 5 min before treatment with PGE2 (500 nM) followed by (e,g) nigericin (5 μM for 30 min) or (f,h) ATP (5 mM 45 min). (a-h). Total IL-1β release in cell supernatants analyzed by ELISA. (b,c,f,h) Immunoblot analysis of culture supernatants (SN) and lysates (LYS). *P < 0.005 (unpaired two-tailed t test). (a-h) Data are from one experiment representative of three separate experiments with similar results, (a-h) BMDM were derived from 2 mice per experiment mixed together, (a-h) three replicates, each replicate is one well + s.e.m.
Supplementary Figure 5 cAMP induces rapid NLRP3 inflammasome inhibition.
(a-c,e,f) BMDMs primed for 3.5 h with LPS. (a,b) The indicated concentration of forskolin was added 5 min prior to (a) nigericin (5 μM for 30 min) or (b) ATP (5 mM for 45 min). (c) db-cAMP (1 μM) was added 5 min prior to nigericin (5 μM for 30 min) or ATP (5 mM for 45 min). (d) PMA-differentiated THP-1 cells were treated for 5 min with indicated concentration of forskolin or db-cAMP prior to addition of nigericin (5 μM) and LPS (50 ng/ml) for 30 min. (e) Forskolin (50 μM) was added 30 min after stimulation with nigericin and analyzed 50 min post initial nigericin stimulation. (f) Forskolin (50 μM) or db-cAMP (1 μM) was added for 5 min prior to nigericin (5 μM for 30 min). (a-e) Total IL-1β release in cell supernatants analyzed by ELISA. (b-d,f) Immunoblot analysis of culture supernatants (SN) and lysates (LYS). *P < 0.005 (unpaired two-tailed t test). (a-f) Data are from one experiment representative of three separate experiments with similar results, (a-c,e,f) BMDM were derived from 2 mice per experiment mixed together, (a-e) three replicates, each replicate is one well + s.e.m.
Supplementary Figure 6 PKA inhibits the NLRP3 inflammasome in response to extracellular agonists but not particulate agonists.
(a-h) BMDMs primed for 3.5 h with LPS. (a,c) The indicated concentration of 6-Bnz-cAMP or (b) 8-CPT-2-O-Me-cAMP was added 5 min before nigericin (5 μM for 30 min). (d) 6-Bnz-cAMP (50 μM) or 8-CPT-2-O-Me-cAMP (50 μM) was added 5 min prior to treatment with MSU (125 μg/ml for 4h) or silica (250 μg/ml for 5h). (e) Rp-cAMP was added 5 min before or (f) PKI was added 30 min before (e,f) 5 min treatment with db-cAMP (1 μM) or forskolin (50 μM) and followed by nigericin (5 μM for 30 min). (g) Rp-cAMP was added 5 min before or (h) PKI was added 30 min before (g,h) 5 min treatment with PGE2 (500 nM) followed by nigericin (5 μM for 30 min). (a,b,d-h) Total IL-1β release in cell supernatants analyzed by ELISA. (c-e) Immunoblot analysis of culture supernatants (SN) and lysates (LYS). *P < 0.005 (unpaired two-tailed t test). (a-h) Data are from one experiment representative of three separate experiments with similar results, (a-h) BMDM were derived from 2 mice per experiment mixed together, (a,b,d-h) three replicates, each replicate is one well + s.e.m.
Supplementary Figure 7 PGE2-induced suppression of nigericin-triggered NLRP3 inflammasomes is mediated by PKA, but particulate NLRP3 agonists are not.
(a-f) BMDMs primed for 3.5 h with LPS. (a) Rp-cAMP was added 5 min before or (b) PKI was added 30 min before treatment with EP2 agonist ONO-AE1-259-01 (50 nM) for 5 min followed by nigericin (5 μM for 30 min). (c) Rp-cAMP was added 5 min before or (d) PKI was added 30 min before treatment with EP4 agonist ONO-AE1-329 (500 nM) for 5 min followed by nigericin (5 μM for 30 min). (e) PKI was added 30 min before or (f) Rp-cAMP was added 5 min before (e,f) PGE2 (500 nM) treatment for 5 min followed by addition of MSU (125 μg/ml for 4h) or silica (250 μg/ml for 5h). (a,c,e,f) Immunoblot analysis of culture supernatants (SN) and lysates (LYS). (a-f) Total IL-1β release in cell supernatants analyzed by ELISA. *P < 0.005 (unpaired two-tailed t test). (a-f) Data are from one experiment representative of three separate experiments with similar results, (a-f) BMDM were derived from 2 mice per experiment mixed together, (a-f) three replicates, each replicate is one well + s.e.m.
Supplementary Figure 8 PKA C-α and NLRP3 interact.
(a) Flow of NLRP3 ATPase assay used in Figure 6e and Figure 7e. (b,c) Immunoprecipitation of indicated protein of BMDMs primed for 3.5 h with LPS and treated for 5 min with forskolin (50 μM) in the presence or absence of PKI (1 μM). (b,c) Data are from one experiment representative of three separate experiments with similar results, (b,c) BMDM were derived from 2 mice per experiment mixed together.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 2223 kb)
Rights and permissions
About this article
Cite this article
Mortimer, L., Moreau, F., MacDonald, J. et al. NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. Nat Immunol 17, 1176–1186 (2016). https://doi.org/10.1038/ni.3538
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3538