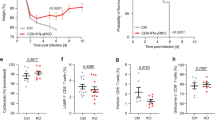
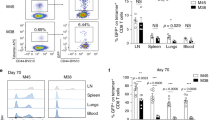
Abstract
Pathogen-specific CD8+ T cells expand in number after infection and then their numbers invariably contract by 90–95%, leaving a stable memory cell pool. The chief features of this response are programmed early after infection; however, the factors regulating contraction are mostly undefined. Here we show that antibiotic treatment before Listeria monocytogenes infection induced numbers of protective memory CD8+ T cells similar to those in control infected mice, by a pathway without contraction. The absence of contraction correlated with decreased early inflammation and interferon-γ production and an increased fraction of CD8+ T cells expressing the interleukin 7 receptor at the peak of the response. Thus, contraction is controlled by early inflammation but is not essential for the generation of protective memory CD8+ T cells after infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Sprent, J. & Surh, C.D. T cell memory. Ann. Rev. Immunol. 20, 551–579 (2002).
Kaech, S.M., Wherry, E.J. & Ahmed, R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2, 251–262 (2002).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Ann. Rev. Immunol. 22, 745–763 (2004).
Harty, J.T., Tvinnereim, A.R. & White, D.W. CD8+ T cell effector mechanisms in resistance to infection. Ann. Rev. Immunol. 18, 275–308 (2000).
Harty, J.T. & Badovinac, V.P. Influence of effector molecules on the CD8+ T cell response to infection. Curr. Opin. Immunol. 14, 360–365 (2002).
Seder, R.A. & Ahmed, R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4, 835–842 (2003).
Prlic, M., Lefrancois, L. & Jameson, S.C. Regulation of memory CD8 T Cell generation and homeostasis by interleukin (IL)-7 and IL-15. J. Exp. Med. 195, F49–F52 (2002).
Schluns, K.S., Williams, K., Ma, A., Zheng, X.X. & Lefrancois, L. Cutting edge: Requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168, 4827–4831 (2002).
Becker, T.C. et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195, 1541–1548 (2002).
Kieper, W.C. et al. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 195, 1533–1539 (2002).
Goldrath, A.W. et al. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195, 1515–1522 (2002).
Tan, J.T. et al. IL-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory-phenotype CD4+ cells. J. Exp. Med. 195, 1523–1532 (2002).
Weninger, W., Crowley, M.A., Manjunath, N. & von Andrian, U.H. Migratory properties of naive, effector, and memory CD8+ T cells. J. Exp. Med. 194, 953–966 (2001).
Wherry, E.J. et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4, 225–234 (2003).
Mercado, R. et al. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165, 6833–6839 (2000).
Kaech, S.M. & Ahmed, R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2, 415–422 (2001).
Wong, P. & Pamer, E.G. Cutting edge: antigen-independent CD8 T cell proliferation. J. Immunol. 166, 5864–5868 (2001).
van Stipdonk, M.J., Lemmens, E.E. & Schoenberger, S.P. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2, 423–429 (2001).
Foulds, K.E. et al. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 168, 1528–1532 (2002).
Badovinac, V.P., Porter, B.B. & Harty, J.T. Programmed contraction of CD8+ T cells after infection. Nat. Immunol. 3, 619–626 (2002).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Hildeman, D.A., Zhu, Y., Mitchell, T.C., Kappler, J. & Marrack, P. Molecular mechanisms of activated T cell death in vivo. Curr. Opin. Immunol. 14, 354–359 (2002).
Plas, D.R., Rathmell, J.C. & Thompson, C.B. Homeostatic control of lymphocyte survival: potential origins and implications. Nat. Immunol. 3, 515–521 (2002).
Badovinac, V.P., Hamilton, S.E. & Harty, J.T. Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity 18, 463–474 (2003).
Wherry, E.J., Blattman, J.N., Murali-Krishna, K., van der Most, R. & Ahmed, R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003).
Fuller, M.J. & Zajac, A.J. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170, 477–486 (2003).
Maraskovsky, E. et al. Impaired survival and proliferation in IL-7 receptor-deficient peripheral T cells. J. Immunol. 157, 5315–5323 (1996).
Vella, A., Teague, T.K., Ihle, J., Kappler, J. & Marrack, P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J. Exp. Med. 186, 325–330 (1997).
Ku, C.C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 288, 675–678 (2000).
Schluns, K.S., Kieper, W.C., Jameson, S.C. & Lefrancois, L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1, 426–432 (2000).
Schluns, K.S. & Lefrancois, L. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3, 269–279 (2003).
Kaech, S.M. et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4, 1191–1198 (2003).
Jacob, J. & Baltimore, D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature 399, 593–597 (1999).
Opferman, J.T., Ober, B.T. & Ashton-Rickardt, P.G. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science 283, 1745–1748 (1999).
North, R.J., Berche, P.A. & Newborg, M.F. Immunologic consequences of antibiotic-induced abridgement of bacterial infection: effect on generation and loss of protective T cells and level of immunologic memory. J. Immunol. 127, 342–346 (1981).
Hou, S., Hyland, L., Ryan, K.W., Portner, A. & Doherty, P.C. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature 369, 652–654 (1994).
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998).
Butz, E.A. & Bevan, M.J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8, 167–175 (1998).
Busch, D.H., Pilip, I.M., Vijh, S. & Pamer, E.G. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8, 353–362 (1998).
Homann, D., Teyton, L. & Oldstone, M.B. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7, 913–919 (2001).
Poston, R.M. & Kurlander, R.J. Analysis of the time course of IFN-γ mRNA and protein production during primary murine listeriosis. The immune phase of bacterial elimination is not temporally linked to IFN production in vivo. J. Immunol. 146, 4333–4337 (1991).
Badovinac, V.P., Tvinnereim, A.R. & Harty, J.T. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-γ. Science 290, 1354–1357 (2000).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Ann. Rev. Immunol. 21, 335–376 (2003).
Krieg, A.M. CpG motifs: the active ingredient in bacterial extracts? Nat. Med. 9, 831–835 (2003).
Jameson, S.C. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2, 547–556 (2002).
Sprent, J. & Surh, C.D. T cell death and memory. Science 293, 245–248 (2001).
Madakamutil, L.T. et al. CD8αα-mediated survival and differentiation of CD8 memory T cell precursors. Science 304, 590–593 (2004).
Bachmann, M.F., Barner, M., Viola, A. & Kopf, M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur. J. Immunol. 29, 291–299 (1999).
Dutton, R.W., Bradley, L.M. & Swain, S.L. T cell memory. Ann. Rev. Immunol. 16, 201–223 (1998).
Veiga-Fernandes, H., Walter, U., Bourgeois, C., McLean, A. & Rocha, B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat. Immunol. 1, 47–53 (2000).
Kaech, S.M., Hemby, S., Kersh, E. & Ahmed, R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111, 837–851 (2002).
Harty, J.T. & Bevan, M.J. Specific immunity to Listeria monocytogenes in the absence of IFN γ. Immunity 3, 109–117 (1995).
Brundage, R.A., Smith, G.A., Camilli, A., Theriot, J.A. & Portnoy, D.A. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc. Nat. Acad. Sci. USA 90, 11890–1184 (1993).
Pamer, E.G., Harty, J.T. & Bevan, M.J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature 353, 852–855 (1991).
van der Most, R.G. et al. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J. Immunol. 157, 5543–5554 (1996).
Badovinac, V.P. & Harty, J.T. Intracellular staining for TNF and IFN-γ detects different frequencies of antigen-specific CD8+ T cells. J. Immunol. Meth. 238, 107–117 (2000).
Acknowledgements
We thank K. Rensberger and R. Podyminogin for technical assistance; R. Ashman and P. Lenert (University of Iowa) for CpG oligonucleotides; and S. Perlman and S. Varga (University of Iowa) for critical review of the manuscript. Supported by National Institutes of Health (AI42767, AI46653 and AI50073 to J.T.H., and T32AI07485 to B.B.P.) and The Leukemia and Lymphoma Society (V.P.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Ex vivo detection of Ag-specific CD8+ T cells after L. monocytogenes infection. (PDF 30 kb)
Supplementary Fig. 2
LCMV-specific CD8+ T cell response is not altered in Amp-pretreated mice. (PDF 32 kb)
Supplementary Fig. 3
Ag-specific CD8+ T cells in low and high dose infected control mice exhibit similar response kinetics and IL-7R expression. (PDF 34 kb)
Supplementary Fig. 4
Increased Bcl-2 expression on Ag-specific CD8+ T cells in Amp-pretreated mice after L. monocytogenes infection. (PDF 40 kb)
Supplementary Fig. 5
Bacterial clearance and lack of contraction in L. monocytogenes infected IFN-γ-deficient mice after Amp treatment. (PDF 31 kb)
Rights and permissions
About this article
Cite this article
Badovinac, V., Porter, B. & Harty, J. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol 5, 809–817 (2004). https://doi.org/10.1038/ni1098
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1098
This article is cited by
-
The interplay of drug therapeutics and immune responses to SARS-CoV-2
Cellular & Molecular Immunology (2023)
-
Pyrimidine de novo synthesis inhibition selectively blocks effector but not memory T cell development
Nature Immunology (2023)
-
Low-dose interleukin-2 can improve salivary secretion but not lymphocyte infiltration of salivary glands in a murine model of Sjögren’s syndrome
BMC Immunology (2022)
-
Interferon-γ: teammate or opponent in the tumour microenvironment?
Nature Reviews Immunology (2022)
-
Differential expansion of T central memory precursor and effector subsets is regulated by division speed
Nature Communications (2020)