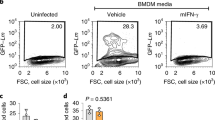
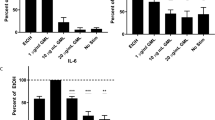
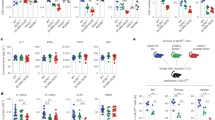
Abstract
T cell–independent type 1 agonists such as Gram-negative bacterial lipopolysaccharides can stimulate B lymphocytes to proliferate and produce antibodies by signaling through Toll-like receptors. This phenomenon is well established in vitro, yet polyclonal B cell responses after bacterial infection in vivo are often weak and short-lived. We show here that B cell proliferation and polyclonal antibody production in response to Gram-negative bacterial infection are modulated by acyloxyacyl hydrolase, a host enzyme that deacylates bacterial lipopolysaccharides. Deacylation of lipopolysaccharide occurred over several days, allowing lipopolysaccharide to act as an innate immune stimulant yet limiting the eventual amount of B cell proliferation and polyclonal antibody production. Control of lipopolysaccharide activation by acyloxyacyl hydrolase indicates that mammals can regulate immune responses to bacterial infection by chemical modification of a Toll-like receptor agonist.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Erwin, A.L. & Munford, R.S. Deacylation of structurally diverse lipopolysaccharides by human acyloxyacyl hydrolase. J. Biol. Chem. 265, 16444–16449 (1990).
Kitchens, R.L., Ulevitch, R.J. & Munford, R.S. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J. Exp. Med. 176, 485–494 (1992).
Munford, R.S. & Hall, C.L. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science 234, 203–205 (1986).
Luchi, M. & Munford, R.S. Binding, internalization, and deacylation of bacterial lipopolysaccharides by human neutrophils. J. Immunol. 151, 959–969 (1993).
Katz, S.S., Weinrauch, Y., Munford, R.S., Elsbach, P. & Weiss, J. Deacylation of lipopolysaccharide in whole Escherichia coli during destruction by cellular and extracellular components of a rabbit inflammatory peritoneal exudate. J. Biol. Chem. 274, 36579–36584 (1999).
Lu, M. et al. Stimulus-dependent deacylation of bacterial lipopolysaccharide by dendritic cells. J. Exp. Med. 197, 1745–1754 (2003).
Ochsenbein, A.F. & Zinkernagel, R. Natural antibodies and complement link innate and acquired immunity. Immunol. Today 21, 624–630 (2000).
Boes, M. Role of natural and immune IgM antibodies in immune responses. Mol. Immunol. 37, 1141–1149 (2000).
Nagai, Y. et al. The radioprotective 105/MD-1 complex links TLR2 and TLR4/MD-2 in antibody response to microbial membranes. J. Immunol. 174, 7043–7049 (2005).
Coutinho, A., Moller, G. & Gronowicz, E. Genetical control of B-cell responses. IV. Inheritance of the unresponsiveness to lipopolysaccharides. J. Exp. Med. 142, 253–258 (1975).
Watson, J. & Riblet, R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic responses to lipopolysaccharides. J. Exp. Med. 140, 1147–1161 (1974).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Krieg, A.M. et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374, 546–549 (1995).
Dziarski, R. Recognition of bacterial peptidoglycan by the innate immune system. Cell. Mol. Life Sci. 60, 1793–1804 (2003).
Massari, P. et al. Cutting edge: Immune stimulation by neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 168, 1533–1537 (2002).
Coutinho, A., Gronowicz, E., Bullock, W.W. & Moller, G. Mechanism of thymus-independent immunocyte triggering. Mitogenic activation of B cells results in specific immune responses. J. Exp. Med. 139, 74–92 (1974).
Dziarski, R. Preferential induction of autoantibody secretion in polyclonal activation by peptidoglycan and lipopolysaccharide. II. In vivo studies. J. Immunol. 128, 1026–1030 (1982).
Dziarski, R. Studies on the mechanism of peptidoglycan- and lipopolysaccharide-induced polyclonal activation. Infect. Immun. 35, 507–514 (1982).
Preston, A., Mandrell, R.E., Gibson, B.W. & Apicella, M.A. The lipooligosaccharides of pathogenic gram-negative bacteria. Crit. Rev. Microbiol. 22, 139–180 (1996).
Erwin, A.L., Mandrell, R.E. & Munford, R.S. Enzymatically deacylated Neisseria LPS inhibits murine splenocyte mitogenesis induced by LPS. Infect. Immun. 59, 1881–1887 (1991).
Van der Ley, P. et al. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 69, 5981–5990 (2001).
Munford, R.S. Detoxifying endotoxin: time, place, person. J. Endotoxin Res. 11, 69–84 (2005).
Cross, A.S. Endotoxin tolerance — current concepts in historical perspective. J. Endotoxin Res. 8, 83–98 (2002).
Liew, F.Y., Xu, D., Brint, E.K. & O'Neill, L.A. Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5, 446–458 (2005).
Upton, C. & Buckley, J.T. A new family of lipolytic enzymes? Trends Biochem. Sci. 20, 178–179 (1995).
Munford, R.S., Sheppard, P.O. & O'Hara, P.J. Saposin-like proteins (SAPLIP) carry out diverse functions on a common backbone structure. J. Lipid Res. 36, 1653–1663 (1995).
Feulner, J.A. et al. Identification of acyloxyacyl hydrolase, a lipopolysaccharide-detoxifying enzyme, in the murine urinary tract. Infect. Immun. 72, 3171–3178 (2004).
Cody, M.J. et al. Effect of inflammatory and anti-inflammatory stimuli on acyloxyacyl hydrolase gene expression and enzymatic activity in murine macrophages. J. Endotoxin Res. 4, 371–379 (1997).
Forestier, C. et al. Interaction of Brucella abortus lipopolysaccharide with major histocompatibility complex class II molecules in B lymphocytes. Infect. Immun. 67, 4048–4054 (1999).
Weinrauch, Y., Katz, S.S., Munford, R.S., Elsbach, P. & Weiss, J. Deacylation of purified LPS by cellular and extracellular components of a sterile rabbit peritoneal inflammatory exudate. Infect. Immun. 67, 3376–3382 (1999).
Snapper, C.M., Rosas, F.R., Kehry, M.R., Mond, J.J. & Wetzler, L.M. Neisserial porins may provide critical second signals to polysaccharide-activated murine B cells for induction of immunoglobulin secretion. Infect. Immun. 65, 3203–3208 (1997).
Bhasin, N., Ho, Y. & Wetzler, L.M. Neissetia meningitides lipopolysaccharide modulates the specific humoral immune response to neisserial porins but has no effect on porin-induced upregulation of costimulatory ligand B7–2. Infect. Immun. 69, 5031–5036 (2001).
Zinkernagel, R. & Hengartner, H. Regulation of the immune response by antigen. Science 293, 251–253 (2001).
Uhr, J.W. & Möller, G. Regulatory effect of antibody on the immune response. Adv. Immunol. 8, 81–127 (1968).
Takai, T., Ono, M., Hikida, M., Ohmori, H. & Ravetch, J.V. Augmented humoral and anaphylactic responses in FcγRII-deficient mice. Nature 379, 346–349 (1996).
Heyman, B. Feedback regulation by IgG antibodies. Immunol. Lett. 88, 157–161 (2003).
Medzhitov, R. & Janeway, C.A. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9, 4–9 (1997).
Martin, F. & Kearney, J.F. B1 cells: similarities and differences with other B cell subsets. Curr. Opin. Immunol. 13, 195–201 (2001).
Beutler, B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430, 257–263 (2004).
Stoll, L.L., Denning, G.M. & Weintraub, N.L. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 24, 2227–2236 (2004).
Douwes, J., Pearce, N. & Heederik, D. Does environmental endotoxin exposure prevent asthma? Thorax 57, 86–90 (2002).
Liu, A.H. Endotoxin exposure in allergy and asthma: Reconciling a paradox. J. Allergy Clin. Immunol. 109, 379–392 (2002).
Nagy, L.E. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp. Biol. Med. 228, 882–890 (2003).
Saito, H. & Ishii, H. Recent understanding of immunological aspects in alcoholic hepatitis. Hepatol. Res. 30, 193–198 (2004).
Giardina, P.C. et al. Construction of acetate auxotrophs of Neisseria meningitidis to study host-meningococcal endotoxin interactions. J. Biol. Chem. 276, 5883–5891 (2001).
Manthey, C.L. & Vogel, S.N. Elimination of trace endotoxin protein from rough chemotype LPS. J. Endotoxin Res. 1, 84–91 (1994).
Munford, R.S. & Erwin, A.L. Eucaryotic lipopolysaccharide deacylating enzyme. Methods Enzymol. 209, 485–492 (1992).
Poltorak, A., Smirnova, I., Clisch, R. & Beutler, B. Limits of a deletion spanning Tlr4 in C57BL/10ScCr mice. J. Endotoxin Res. 6, 51–56 (2000).
Wilder, J.A., Koh, C.Y. & Yuan, D. The role of NK cells during In vivo antigen-specific antibody responses. J. Immunol. 156, 146–152 (1996).
Mohan, C., Adams, S., Stanik, V. & Datta, S.K. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J. Exp. Med. 177, 1367–1381 (1993).
Acknowledgements
We thank R. Kitchens, R. Dziarski and L. Wetzler for advice. Supported by the National Institutes of Health (AI18188 and AI44642) and by the Jan and Henri Bromberg Chair in Internal Medicine (The University of Texas Southwestern Medical Center).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lu, M., Zhang, M., Takashima, A. et al. Lipopolysaccharide deacylation by an endogenous lipase controls innate antibody responses to Gram-negative bacteria. Nat Immunol 6, 989–994 (2005). https://doi.org/10.1038/ni1246
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1246
This article is cited by
-
The gut–liver axis in sepsis: interaction mechanisms and therapeutic potential
Critical Care (2022)
-
Novel insights in preventing Gram-negative bacterial infection in cirrhotic patients: review on the effects of GM-CSF in maintaining homeostasis of the immune system
Hepatology International (2015)
-
A high-throughput, multiplexed assay for superfamily-wide profiling of enzyme activity
Nature Chemical Biology (2014)