Abstract
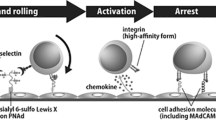
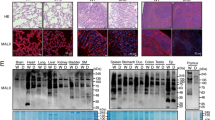
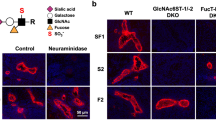
Lymphocyte homing is mediated by specific interactions between L-selectin on lymphocytes and sulfated carbohydrates restricted to high endothelial venules in lymph nodes. Here we generated mice deficient in both N-acetylglucosamine-6-O-sulfotransferase 1 (GlcNAc6ST-1) and GlcNAc6ST-2 and found that mutant mice had approximately 75% less homing of lymphocytes to the peripheral lymph nodes than did wild-type mice. Consequently, these mice had lower contact hypersensitivity responses than those of wild-type mice. Carbohydrate structural analysis showed that 6-sulfo sialyl Lewis X, a dominant ligand for L-selectin, was almost completely absent from the high endothelial venules of these mutant mice, whereas the amount of unsulfated sialyl Lewis X was much greater. These results demonstrate the essential function of GlcNAc6ST-1 and GlcNAc6ST-2 in L-selectin ligand biosynthesis in high endothelial venules and their importance in immune surveillance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Arbonés, M.L. et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity 1, 247–260 (1994).
Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76, 301–314 (1994).
Butcher, E.C. & Picker, L.J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
von Andrian, U.H. & Mempel, T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3, 867–878 (2003).
Ley, K. & Kansas, G.S. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 4, 325–335 (2004).
Rosen, S.D. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 22, 129–156 (2004).
Maly, P. et al. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86, 643–653 (1996).
Homeister, J.W. et al. The α(1,3) fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity 15, 115–126 (2001).
Rosen, S.D., Singer, M.S. & Yednock, T.A. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science 228, 1005–1007 (1985).
Imai, Y., Lasky, L.A. & Rosen, S.D. Sulphation requirement for GlyCAM-1, an endothelial ligand for L-selectin. Nature 361, 555–557 (1993).
Hemmerich, S., Butcher, E.C. & Rosen, S.D. Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA-79, an adhesion-blocking monoclonal antibody. J. Exp. Med. 180, 2219–2226 (1994).
Mitsuoka, C. et al. Identification of a major carbohydrate capping group of the L-selectin ligand on high endothelial venules in human lymph nodes as 6-sulfo sialyl Lewis X. J. Biol. Chem. 273, 11225–11233 (1998).
Hiraoka, N. et al. A novel, high endothelial venule-specific sulfotransferase expresses 6-sulfo sialyl Lewisx, an L-selectin ligand displayed by CD34. Immunity 11, 79–89 (1999).
Bistrup, A. et al. Sulfotransferases of two specificities function in the reconstitution of high endothelial cell ligands for L-selectin. J. Cell Biol. 145, 899–910 (1999).
Yeh, J.-C. et al. Novel sulfated lymphocyte homing receptors and their control by a core1 extension β1,3-N-acetylglucosaminyltransferase. Cell 105, 957–969 (2001).
Streeter, P.R., Rouse, B.T.N. & Butcher, E.C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J. Cell Biol. 107, 1853–1862 (1988).
Hiraoka, N. et al. Core 2 branching β1,6-N-acetylglucosaminyltransferase and high endothelial venule-restricted sulfotransferase collaboratively control lymphocyte homing. J. Biol. Chem. 279, 3058–3067 (2004).
Hemmerich, S. et al. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity 15, 237–247 (2001).
Fukuda, M., Hiraoka, N., Akama, T.O. & Fukuda, M.N. Carbohydrate-modifying sulfotransferases: structure, function and pathophysiology. J. Biol. Chem. 276, 47747–47750 (2001).
Hemmerich, S. et al. Chromosomal localization and genomic organization for the galactose/N-acetylgalactosamine/N-acetylglucosamine 6-O-sulfotransferase gene family. Glycobiology 11, 75–87 (2001).
Uchimura, K. et al. Molecular cloning and characterization of an N-acetylglucosamine-6-O-sulfotransferase. J. Biol. Chem. 273, 22577–22583 (1998).
Kimura, N. et al. Reconstitution of functional L-selectin ligands on a cultured human endothelial cell line by cotransfection of α1–3 fucosyltransferase VII and newly cloned GlcNAcβ:6-sulfotransferase cDNA. Proc. Natl. Acad. Sci. USA 96, 4530–4535 (1999).
Uchimura, K. et al. N-Acetylglucosamine 6-O-sulfotransferase-1 regulates expression of L-selectin ligands and lymphocyte homing. J. Biol. Chem. 279, 35001–35008 (2004).
Berlin, C. et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993).
Phanuphak, P., Moorhead, J.W. & Claman, H.N. Tolerance and contact sensitivity to DNFB in mice. I. In vivo detection by ear swelling and correlation with in vitro cell stimulation. J. Immunol. 112, 115–123 (1974).
Uchimura, K. et al. Diversity of N-acetylglucosamine-6-O-sulfotransferases: molecular cloning of a novel enzyme with different distribution and specificities. Biochem. Biophys. Res. Commun. 274, 291–296 (2000).
Bhakta, S. et al. Sulfation of N-acetylglucosamine by chondroitin 6-sulfotransferase 2 (GST-5). J. Biol. Chem. 275, 40226–40234 (2000).
Lee, J.K., Bhakta, S., Rosen, S.D. & Hemmerich, S. Cloning and characterization of a mammalian N-acetylglucosamine-6-sulfotransferase that is highly restricted to intestinal tissue. Biochem. Biophys. Res. Commun. 263, 543–549 (1999).
Akama, T.O. et al. Human corneal GlcNAc 6-O-sulfotransferase and mouse intestinal GlcNAc 6-O-sulfotransferase both produce keratan sulfate. J. Biol. Chem. 276, 16271–16278 (2001).
Fukuta, M. et al. Molecular cloning and characterization of human keratan sulfate Gal-6-sulfotransferase. J. Biol. Chem. 272, 32321–32328 (1997).
de Graffenried, C.L. & Bertozzi, C.R. Golgi localization of carbohydrate sulfotransferases is a determinant of L-selectin ligand biosynthesis. J. Biol. Chem. 278, 40282–40295 (2003).
Zerfaoui, M. et al. The cytosolic and transmembrane domains of the β1,6 N-acetylglucosaminyltransferase (C2GnT) function as a cis to medial/Golgi-targeting determinant. Glycobiology 12, 15–24 (2002).
Foxall, C. et al. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewisx oligosaccharide. J. Cell Biol. 117, 895–902 (1992).
Mitoma, J. et al. Extended core 1 and core 2 branched O-glycans differentially modulate sialyl Lewis x-type L-selectin ligand activity. J. Biol. Chem. 278, 9953–9961 (2003).
Fieger, C.B., Sassetti, C.M. & Rosen, S.D. Endoglycan, a member of the CD34 family, functions as an L-selectin ligand through modification with tyrosine sulfation and sialyl Lewis x. J. Biol. Chem. 278, 27390–27398 (2003).
Finger, E.B. et al. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature 379, 266–269 (1996).
Tangemann, K., Bistrup, A., Hemmerich, S. & Rosen, S.D. Sulfation of a high endothelial venule-expressed ligand for L-selectin: effects on tethering and rolling of lymphocytes. J. Exp. Med. 190, 935–941 (1999).
Uchimura, K. et al. A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat. Immunol. advance online publication 9 October 2005(1038/ni1258).
M'Rini, C. et al. A Novel endothelial L-selectin ligand activity in lymph node medulla that is regulated by α(1,3)-fucosyltransferase-IV. J. Exp. Med. 198, 1301–1312 (2003).
Hemmerich, S., Leffler, H. & Rosen, S.D. Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J. Biol. Chem. 270, 12035–12047 (1995).
Torii, T., Fukuta, M. & Habuchi, O. Sulfation of sialyl N-acetyllactosamine oligosaccharides and fetuin oligosaccharides by keratan sulfate Gal-6-sulfotransferase. Glycobiology 10, 203–211 (2000).
Catalina, M.D. et al. The route of antigen entry determines the requirement for L-selectin during immune responses. J. Exp. Med. 184, 2341–2351 (1996).
Smithson, G. et al. Fuc-TVII is required for T helper 1 and T cytotoxic 1 lymphocyte selectin ligand expression and recruitment in inflammation, and together with Fuc-TIV regulates naive T cell trafficking to lymph nodes. J. Exp. Med. 194, 601–614 (2001).
Rosen, S.D. Endothelial ligands for L-selectin. From lymphocyte recirculation to allograft rejection. Am. J. Pathol. 155, 1013–1020 (1999).
Kobayashi, M. et al. Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 101, 17807–17812 (2004).
Rosen, S.D., Tsay, D., Singer, M.S., Hemmerich, S. & Abraham, W.M. Therapeutic targeting of endothelial ligands for L-selectin (PNAd) in a sheep model of asthma. Am. J. Pathol. 166, 935–944 (2005).
Hanninen, A. et al. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J. Clin. Invest. 92, 2509–2515 (1993).
Faveeuw, C., Gagnerault, M-C. & Lepault, F. Expression of homing and adhesion molecules in infiltrated islets of Langerhans and salivary glands of nonobese diabetic mice. J. Immunol. 152, 5969–5978 (1994).
Michie, S.A., Streeter, P.R., Butcher, E.C. & Rouse, R.V. L-selectin and α4β7 integrin homing receptor pathways mediate peripheral lymphocyte traffic to AKR mouse hyperplastic thymus. Am. J. Pathol. 147, 412–421 (1995).
Michie, S.A., Streeter, P.R., Bolt, P.A., Butcher, E.C. & : Picker, L.J. The human peripheral lymph node vascular addressin. An inducible endothelial antigen involved in lymphocyte homing. Am. J. Pathol. 143, 1688–1698 (1993).
Fox, J.G. et al. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology 110, 155–166 (1996).
Singer, M.S. & Rosen, S.D. Purification and quantification of L-selectin-reactive GlyCAM-1 from mouse serum. J. Immunol. Methods 196, 153–161 (1996).
Girard, J.P. & Springer, T.A. Cloning from purified high endothelial venule cells of hevin, a close relative of the antiadhesive extracellular matrix protein SPARC. Immunity 2, 113–123 (1995).
Deutscher, S.L., Nuwayhid, N., Stanley, P., Briles, E.I. & Hirschberg, C.B. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell 39, 295–299 (1984).
Acknowledgements
We thank T. Akama, S. Chen and E. Lammar for critical reading of the manuscript, and A. Morse for organizing the manuscript. Supported by the National Institutes of Health (P01CA71932 to M.F. and J.B.L.; U54 GM62116 to the Functional Glycomics Consortium) and the Uehara Memorial Foundation, Japan (H.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Preparation and analysis of O-glycans attached to GlyCAM-1. (PDF 1711 kb)
Supplementary Fig. 2
HPLC analysis of sulfated O-glycans attached to GlyCAM-1. (PDF 675 kb)
Supplementary Fig. 3
Structures of unsulfated (0S), monosulfated (1S), and disulfated (2S) O-glycans on a disaccharide core (Di), tetrasaccharide core (Tetra), or hexasaccharide core (Hexa) structure attached to GlyCAM-1 from wild-type (WT), GlcNAc6ST-1-deficient (GlcNAc6ST-1 KO), GlcNAc6ST-2-deficient (GlcNAc6ST-2 KO), and double-deficient (DKO) mice. (PDF 356 kb)
Rights and permissions
About this article
Cite this article
Kawashima, H., Petryniak, B., Hiraoka, N. et al. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat Immunol 6, 1096–1104 (2005). https://doi.org/10.1038/ni1259
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1259
This article is cited by
-
Immunomodulation by endothelial cells — partnering up with the immune system?
Nature Reviews Immunology (2022)
-
High endothelial venules (HEVs) in immunity, inflammation and cancer
Angiogenesis (2021)
-
Preferential expression of sialyl 6′-sulfo N-acetyllactosamine-capped O-glycans on high endothelial venules in human peripheral lymph nodes
Laboratory Investigation (2019)
-
MyD88 signaling causes autoimmune sialadenitis through formation of high endothelial venules and upregulation of LTβ receptor-mediated signaling
Scientific Reports (2018)
-
Cytosolic sulfotransferase 1A1 regulates HIV-1 minus-strand DNA elongation in primary human monocyte-derived macrophages
Virology Journal (2016)