Abstract
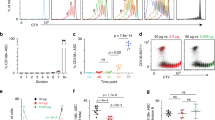
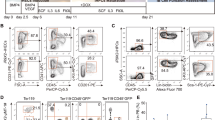
The B-1 subpopulation of B lymphocytes differs phenotypically and functionally from conventional B-2 B cells. B-1 B cells are proposed to derive from a distinct progenitor, but such a population has not been isolated. Here we identify and characterize a B-1 B cell progenitor whose numbers peaked in fetal bone marrow but were less abundant in postnatal bone marrow. These Lin−CD45Rlo–negCD19+ cells responded to thymic stromal lymphopoietin and 'preferentially' reconstituted functional sIgMhiCD11b+CD5lo–neg B-1 B cells, but not sIgM+CD11b− B-2 B cells, in vivo. These data indicate that the CD45Rlo–negCD19+ population includes B-1 B cell–specified progenitors and support models proposing distinct developmental pathways for B-1 B cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dzierzak, E. & Medvinsky, A. in Molecular Biology of B and T-cell Development (eds. Monroe, J.G. and Rothenberg, E.V.) 3–25 (Humana, Totawa, New Jersey, 1998).
Li, Y., Wasserman, R., Hayakawa, K. & Hardy, R. Identification of the earliest B lineage stage in mouse bone marrow. Immunity 5, 527–535 (1996).
Tudor, K., Payne, K.J., Yamashita, Y. & Kincade, P.W. Functional assessment of precursors from murine bone marrow suggests a sequence of early B lineage differentiation events. Immunity 12, 335–345 (2000).
Hardy, R.R., Carmack, C.E., Shinton, S.A., Kemp, J.D. & Hayakawa, K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 173, 1213–1225 (1991).
Allman, D., Ferguson, S. & Cancro, M. Peripheral B cell maturation. I. Immature peripheral B cells in adults are heat-stable antigenhi and exhibit unique signaling characteristics. J. Immunol. 149, 2533–2540 (1992).
Lopes-Carvalho, T. & Kearney, J.F. Development and selection of marginal zone B cells. Immunol. Rev. 197, 192–205 (2004).
Martin, F., Oliver, A.M. & Kearney, J.F. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14, 617–629 (2001).
Hardy, R.R. & Hayakawa, K. B cell development pathways. Annu. Rev. Immunol. 19, 595–621 (2001).
Herzenberg, L.A. B-1 cells: the lineage question revisited. Immunol. Rev. 175, 9–22 (2000).
Hayakawa, K., Hardy, R.R. & Herzenberg, L.A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 161, 1554–1568 (1985).
Lalor, P.A., Stall, A.M., Adams, S. & Herzenberg, L.A. Permanent alteration of the murine Ly-1 B repertoire due to selective depletion of Ly-1 B cells in neonatal animals. Eur. J. Immunol. 19, 501–506 (1989).
Kantor, A.B. & Herzenberg, L.A. Origin of murine B cell lineages. Annu. Rev. Immunol. 11, 501–538 (1993).
Stall, A., Adams, S., Herzenberg, L. & Kantor, A. Characteristics and development of the murine B-1b (Ly-1 B sister) cell population. Ann. NY Acad. Sci. 651, 33–43 (1992).
Herzenberg, L.A. et al. The Ly-1 B cell lineage. Immunol. Rev. 93, 81–102 (1986).
Haas, K.M., Poe, J.C., Steeber, D.A. & Tedder, T.F. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23, 7–18 (2005).
Alugupalli, K.R. et al. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21, 379–390 (2004).
Berland, R. & Wortis, H.H. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20, 253–300 (2002).
Lam, K-P. & Rajewsky, K. B cell antigen receptor specificity and surface density together determine B-1 versus B-2 cell development. J. Exp. Med. 190, 471–478 (1999).
Hardy, R.R. & Hayakawa, K. A developmental switch in B lymphopoiesis. Proc. Natl. Acad. Sci. USA 88, 11550–11554 (1991).
Vosshenrich, C., Cumano, A., Muller, W., Di Santo, J. & Vieria, P. Thymic-stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nat. Immunol. 4, 773–779 (2003).
Carvalho, T.L., Mota-Santos, T., Cumano, A., Demengeot, J. & Vieira, P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7−/− mice. J. Exp. Med. 194, 1141–1150 (2001).
Vosshenrich, C.A., Cumano, A., Muller, W., Di Santo, J.P. & Vieira, P. Pre-B cell receptor expression is necessary for thymic stromal lymphopoietin responsiveness in the bone marrow but not in the liver environment. Proc. Natl. Acad. Sci. USA 101, 11070–11075 (2004).
Park, L.S. et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 192, 659–670 (2000).
Yang, Y-G., deGoma, E., Barth, R.K., Sergio, J.J. & Sykes, M. B-cell reconstitution and xenoreactive anti-pig natural antibody production in severe combined immunodeficient mice reconstituted with immunocompetent B cells from varying sources. Transplant 66, 89–95 (1998).
Kantor, A., Stall, A., Adams, S. & Herzenberg, L. Differential development of progenitor activity for three B-cell lineages. Proc. Natl. Acad. Sci. USA 89, 3320–3324 (1992).
Montecino-Rodriguez, E., Leathers, H. & Dorshkind, K. Bipotential B-macrophage progenitors are present in adult bone marrow. Nat. Immunol. 2, 83–88 (2001).
Borrello, M.A. & Phipps, R.P. The B/macrophage cell — an elusive link between CD5+ B lymphocytes and macrophages. Immunol. Today 17, 471–475 (1996).
Johnson, P., Greenbaum, L., Bottomly, K. & Trowbridge, I. Identification of the alternatively spliced exons of murine CD45 (T200) required for reactivity with B220 and other T200-restricted antibodies. J. Exp. Med. 169, 1179–1184 (1989).
Sudo, T. et al. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl. Acad. Sci. USA 90, 9125–9129 (1993).
Oliver, A., Martin, F., Gartland, G., Carter, R. & Kearney, J. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur. J. Immunol. 27, 2366–2374 (1997).
Carpino, N. et al. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol. Cell. Biol. 24, 2584–2592 (2004).
de Andres, B. et al. The first 3 days of B-cell development in the mouse embryo. Blood 100, 4074–4081 (2002).
Seidl, K.J. et al. Predominant VH genes expressed in innate antibodies are associated with distinctive antigen-binding sites. Proc. Natl. Acad. Sci. USA 96, 2262–2267 (1999).
Knoops, L., Louahed, J. & Renauld, J-C. IL-9-Induced expansion of B-1b cells restores numbers but not function of B-1 lymphocytes in xid mice. J. Immunol. 172, 6101–6106 (2004).
Hardy, R.R. et al. B-cell commitment, development and selection. Immunol. Rev. 175, 23–32 (2000).
Pelayo, R. et al. Lymphoid progenitors and primary routes to becoming cells of the immune system. Curr. Opin. Immunol. 17, 100–107 (2005).
Nishimura, H., Hattori, S., Abe, M., Hirose, S. & Shirai, T. Differential expression of a CD45R epitope(6B2) on murine CD5+ B cells: possible difference in the post-translational modification of CD45 molecules. Cell. Immunol. 140, 432–443 (1992).
Friend, S. et al. A thymic stromal cell line supports in vitro development of surface IgM+ cells and produces a novel growth factor affecting B and T lineage cells. Exp. Hematol. 22, 321–328 (1994).
Levin, S.D. et al. Thymic Stromal Lymphopoietin: A cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J. Immunol. 162, 677–683 (1999).
Adolfsson, J. et al. Identification of Flt3+ lympho-lyeloid stem cells lacking erythro-megakaryocytic potential: A revised road map for adult blood lineage commitment. Cell 121, 295–306 (2005).
Engel, P. et al. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity 3, 39–50 (1995).
Sato, S., Steeber, D.A. & Tedder, T.F. The CD19 signal transduction molecule is a response regulator of B-lymphocyte differentiation. Proc. Natl. Acad. Sci. USA 92, 11558–11562 (1995).
Sato, S., Ono, N., Steeber, D., Pisetsky, D. & Tedder, T. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J. Immunol. 157, 4371–4378 (1996).
Nutt, S., Urbanek, P., Rolink, A. & Busslinger, M. Essential functions of Pax5(BSAP) in pro-B cell development: Difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes Dev. 11, 476–491 (1997).
Collins, L.S. & Dorshkind, K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J. Immunol. 138, 1082–1087 (1987).
Schmitt, T.M. & Zuniga-Pflucker, J.C. Induction of T cell development from hematopoietic progenitor cells by Delta-like-1 in vitro. Immunity 17, 749–756 (2002).
Schlissel, M.S., Corcoran, L.M. & Baltimore, D. Virus-transformed pre-B cells show ordered activation but not inactivation of immunoglobulin gene rearrangement and transcription. J. Exp. Med. 173, 711–720 (1991).
Sap, J., D'Eustachio, P., Givol, D. & Schlessinger, J. Cloning and expression of a widely expressed receptor tyrosine phosphatase. Proc. Natl. Acad. Sci. USA 87, 6112–6116 (1990).
Acknowledgements
We thank M. Teitell and O. Witte for critical reading of this manuscript. Supported by the National Institutes of Health (AI21256 and HL54850). Four-color flow cytometry analysis and cell sorting were done in the Flow Cytometry Core Facility at the University of California at Los Angeles that is supported by the National Institutes of Health (CA16042).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Lin−CD45lo/−CD19+ cells are not contaminated with mature sIgM+ cells. (PDF 142 kb)
Supplementary Fig. 2
Distribution of Lin−CD45Rlo/−CD19+AA4.1+ and Lin−CD45R+CD19−AA4.1+ cells in the liver of embryos of different ages. (PDF 126 kb)
Rights and permissions
About this article
Cite this article
Montecino-Rodriguez, E., Leathers, H. & Dorshkind, K. Identification of a B-1 B cell–specified progenitor. Nat Immunol 7, 293–301 (2006). https://doi.org/10.1038/ni1301
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1301
This article is cited by
-
Age-related accumulation of B-1 cell progenitors in mice reflects changes in miR15a/16-1 expression and radioresistance capacity
Experimental Hematology & Oncology (2023)
-
Antibodies in action: the role of humoral immunity in the fight against atherosclerosis
Immunity & Ageing (2022)
-
A primitive type of renin-expressing lymphocyte protects the organism against infections
Scientific Reports (2021)
-
B1a and B2 cells are characterized by distinct CpG modification states at DNMT3A-maintained enhancers
Nature Communications (2021)
-
Immune Memory in Aging: a Wide Perspective Covering Microbiota, Brain, Metabolism, and Epigenetics
Clinical Reviews in Allergy & Immunology (2021)