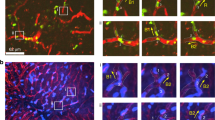
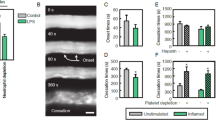
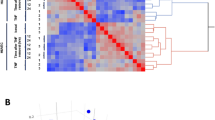
Abstract
Fever is an evolutionarily conserved response during acute inflammation, although its physiological benefit is poorly understood. Here we show thermal stress in the range of fever temperatures increased the intravascular display of two 'gatekeeper' homing molecules, intercellular adhesion molecule 1 (ICAM-1) and CCL21 chemokine, exclusively in high endothelial venules (HEVs) that are chief portals for the entry of blood-borne lymphocytes into lymphoid organs. Enhanced endothelial expression of ICAM-1 and CCL21 was linked to increased lymphocyte trafficking across HEVs. A bifurcation in the mechanisms controlling HEV adhesion was demonstrated by evidence that the thermal induction of ICAM-1 but not of CCL21 involved an interleukin 6 trans-signaling pathway. Our findings identify the 'HEV axis' as a thermally sensitive alert system that heightens immune surveillance during inflammation by amplifying lymphocyte trafficking to lymphoid organs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
10 November 2006
In the version of this article initially published online, the label for the bottom row of Figure 8d is missing. It should read ‘H-IL-6’. The error has been corrected for all versions of the article.
References
Girard, J.P. & Springer, T.A. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol. Today 16, 449–457 (1995).
Butcher, E.C. & Picker, L.J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
von Andrian, U.H. & Mempel, T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3, 867–878 (2003).
Miyasaka, M. & Tanaka, T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat. Rev. Immunol. 4, 360–370 (2004).
Rosen, S.D. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 22, 129–156 (2004).
Engelhardt, B. & Wolburg, H. Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur. J. Immunol. 34, 2955–2963 (2004).
Kluger, M.J. Fever: role of pyrogens and cryogens. Physiol. Rev. 71, 93–127 (1991).
Hasday, J.D., Fairchild, K.D. & Shanholtz, C. The role of fever in the infected host. Microbes Infect. 2, 1891–1904 (2000).
Appenheimer, M.M., Chen, Q., Girard, R., Wang, W.C. & Evans, S.S. Impact of fever-range thermal stress on lymphocyte-endothelial adhesion and lymphcoyte trafficking. Immunol. Invest. 34, 295–323 (2005).
Ostberg, J.R. & Repasky, E.A. Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol. Immunother. 55, 292–298 (2006).
Wang, W.C. et al. Fever-range hyperthermia enhances L-selectin-dependent adhesion of lymphocytes to vascular endothelium. J. Immunol. 160, 961–969 (1998).
Evans, S.S., Bain, M.D. & Wang, W.C. Fever-range hyperthermia stimulates α4β7 integrin-dependent lymphocyte-endothelial adhesion. Int. J. Hyperthermia 16, 45–59 (2000).
Chen, Q. et al. Central role of IL-6 receptor signal-transducing chain gp130 in activation of L-selectin adhesion by fever-range thermal stress. Immunity 20, 59–70 (2004).
Jones, S.A. Directing transition from innate to acquired immunity: defining a role for IL-6. J. Immunol. 175, 3463–3468 (2005).
Jones, S.A. & Rose-John, S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim. Biophys. Acta 1592, 251–263 (2002).
Evans, S.S. et al. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood 97, 2727–2733 (2001).
Chen, Q., Fisher, D.T., Kucinska, S.A., Wang, W.C. & Evans, S.S. Dynamic control of lymphocyte trafficking by fever-range thermal stress. Cancer Immunol. Immunother. 55, 299–311 (2006).
Shah, A. et al. Cytokine and adhesion molecule expression in primary human endothelial cells stimulated with fever-range hyperthermia. Int. J. Hyperthermia 18, 534–551 (2002).
Hasday, J.D. et al. Exposure to febrile temperature modifies endothelial cell response to tumor necrosis factor-α. J. Appl. Physiol. 90, 90–98 (2001).
Choi, J., Enis, D.R., Koh, K.P., Shiao, S.L. & Pober, J.S. T lymphocyte-endothelial cell interactions. Annu. Rev. Immunol. 22, 683–709 (2004).
Gauguet, J.M., Rosen, S.D., Marth, J.D. & von Andrian, U.H. Core 2 branching β1,6-N-acetylglucosaminyltransferase and high endothelial cell N-acetylglucosamine-6-sulfotransferase exert differential control over B- and T-lymphocyte homing to peripheral lymph nodes. Blood 104, 4104–4112 (2004).
Stevens, S.K., Weissman, I.L. & Butcher, E.C. Differences in the migration of B and T lymphocytes: organ-selective localization in vivo and the role of lymphocyte-endothelial cell recognition. J. Immunol. 128, 844–851 (1982).
von Andrian, U.H. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation 3, 287–300 (1996).
Kashiwazaki, M. et al. A high endothelial venule-expressing promiscuous chemokine receptor DARC can bind inflammatory, but not lymphoid, chemokines and is dispensable for lymphocyte homing under physiological conditions. Int. Immunol. 15, 1219–1227 (2003).
Izawa, D. et al. Expression profile of active genes in mouse lymph node high endothelial cells. Int. Immunol. 11, 1989–1998 (1999).
Okada, T. et al. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J. Exp. Med. 196, 65–75 (2002).
Lehmann, J.C. et al. Overlapping and selective roles of endothelial intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 in lymphocyte trafficking. J. Immunol. 171, 2588–2593 (2003).
Gerwin, N. et al. Prolonged eosinophil accumulation in allergic lung interstitium of ICAM-2 deficient mice results in extended hyperresponsiveness. Immunity 10, 9–19 (1999).
McEvoy, L.M., Jutila, M.A., Tsao, P.S., Cooke, J.P. & Butcher, E.C. Anti-CD43 inhibits monocyte-endothelial adhesion in inflammation and atherogenesis. Blood 90, 3587–3594 (1997).
Roebuck, K.A. & Finnegan, A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J. Leukoc. Biol. 66, 876–888 (1999).
Modur, V., Li, Y., Zimmerman, G.A., Prescott, S.M. & McIntyre, T.M. Retrograde inflammatory signaling from neutrophils to endothelial cells by soluble interleukin-6 receptor α. J. Clin. Invest. 100, 2752–2756 (1997).
Romano, M. et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6, 315–325 (1997).
Bergo, M., Wu, G., Ruge, T. & Olivecrona, T. Down-regulation of adipose tissue lipoprotein lipase during fasting requires that a gene, separate from the lipase gene, is switched on. J. Biol. Chem. 277, 11927–11932 (2002).
Fischer, M. et al. I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat. Biotechnol. 15, 142–145 (1997).
Ostberg, J.R., Gellin, C., Patel, R. & Repasky, E.A. Regulatory potential of fever-range whole body hyperthermia on Langerhans cells and lymphocytes in an antigen-dependent cellular immune response. J. Immunol. 167, 2666–2670 (2001).
Sallusto, F. & Mackay, C.R. Chemoattractants and their receptors in homeostasis and inflammation. Curr. Opin. Immunol. 16, 724–731 (2004).
Mackay, C.R., Marston, W. & Dudler, L. Altered patterns of T cell migration through lymph nodes and skin following antigen challenge. Eur. J. Immunol. 22, 2205–2210 (1992).
Tedla, N. et al. Regulation of T lymphocyte trafficking into lymph nodes during an immune response by the chemokines macrophage inflammatory protein (MIP)-1α and MIP-1β. J. Immunol. 161, 5663–5672 (1998).
Soderberg, K.A. et al. Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc. Natl. Acad. Sci. USA 102, 16315–16320 (2005).
Carman, C.V. & Springer, T.A. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr. Opin. Cell Biol. 15, 547–556 (2003).
Shamri, R. et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat. Immunol. 6, 497–506 (2005).
Pachynski, R.K., Wu, S.W., Gunn, M.D. & Erle, D.J. Secondary lymphoid-tissue chemokine (SLC) stimulates integrin α4β7-mediated adhesion of lymphocytes to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) under flow. J. Immunol. 161, 952–956 (1998).
Reilly, P.L. et al. The native structure of intercellular adhesion molecule-1 (ICAM-1) is a dimer. Correlation with binding to LFA-1. J. Immunol. 155, 529–532 (1995).
Miller, J. et al. Intercellular adhesion molecule-1 dimerization and its consequences for adhesion mediated by lymphocyte function associated-1. J. Exp. Med. 182, 1231–1241 (1995).
Casasnovas, J.M., Stehle, T., Liu, J.H., Wang, J.H. & Springer, T.A. A dimeric crystal structure for the N-terminal two domains of intercellular adhesion molecule-1. Proc. Natl. Acad. Sci. USA 95, 4134–4139 (1998).
Sarantos, M.R., Raychaudhuri, S., Lum, A.F., Staunton, D.E. & Simon, S.I. Leukocyte function-associated antigen 1-mediated adhesion stability is dynamically regulated through affinity and valency during bond formation with intercellular adhesion molecule-1. J. Biol. Chem. 280, 28290–28298 (2005).
Carman, C.V. & Springer, T.A. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 167, 377–388 (2004).
Shaw, S.K. et al. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J. Exp. Med. 200, 1571–1580 (2004).
Naka, T., Nishimoto, N. & Kishimoto, T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 4, S233–S242 (2002).
Weninger, W., Crowley, M.A., Manjunath, N. & von Andrian, U.H. Migratory properties of naive, effector, and memory CD8+ T cells. J. Exp. Med. 194, 953–966 (2001).
Abramoff, M.D., Magelhaes, P.J. & Ram, S.J. Image processing with ImageJ. Biophotonics Int. 11, 36–42 (2004).
Acknowledgements
We thank J.D. Black and E.A. Repasky for discussions and comments on the manuscript; M. Miyasaka and T. Tanaka (Osaka University) for antiserum to mouse Duffy antigen–related receptor for chemokines; P.K. Wallace and E.A. Timm for assistance with flow cytometry of leukocyte subsets; and E.L. Hurley for technical support for confocal microscopy. Supported by the US National Institutes of Health (CA79765 and CA094045 to S.S.E.; AI061663 and AI069259 to U.V.A.; DK33886 and CA85580 to H.B.; and CA16056 to Roswell Park Cancer Institute), the Department of Defense (W81XWH-04-1-0354 to Q.C.), the Roswell Park Alliance Foundation (to S.S.E.), the Leukocyte Migration Core of the Harvard Skin Disease Research Center (P30 AR 42689 to U.H.v.A.) and the Deutsche Forschungsgemeinschaft (Bonn, Germany; SFB414, TPB5 to S.R.J.).
Author information
Authors and Affiliations
Contributions
Q.C. and S.S.E. conceptualized and designed the research; S.S.E. supervised the research; Q.C. did all experiments unless stated otherwise; D.T.F. contributed to the experimental design for quantitative image analysis and did the phenotypic analysis in short-term homing assays and enzyme-linked immunosorbent assay for ICAM-1; K.A.C assisted in immunofluorescence staining and kinetic analysis in short-term homing assays; E.U. contributed to the analysis of ICAM-1 staining; W.-C.W. did frozen-section adherence assays and provided technical assistance for organ retrieval; U.H.v.A. and J.-M.G. helped with intravital microscopy studies; S.R.J. provided the hyper-IL-6 expression construct; H.B. provided recombinant hyper-IL-6 and contributed to discussions regarding IL-6 regulation of lymphocyte trafficking; and all authors contributed to discussions and to the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Fever-range thermal stress enhances lymphocyte homing to lymphoid organs with HEV structures without altering the cellular composition of cells recruited into PLNs. (PDF 107 kb)
Supplementary Fig. 2
Fever-range thermal stress increases lymphocyte-endothelial interactions and ICAM-1 expression in PP HEVs. (PDF 3098 kb)
Supplementary Fig. 3
Fever-range thermal stress did not change total protein abundance of ICAM-1 or CCL21 expression in PLNs. (PDF 155 kb)
Supplementary Fig. 4
ICAM-1 is required for thermal stimulation of lymphocyte trafficking. (PDF 115 kb)
Supplementary Fig. 5
Model for the molecular mechanisms underlying thermal stimulation of lymphocyte trafficking. (PDF 99 kb)
Supplementary Table 1
Summary of vascular adhesion molecule expression in different organs. (PDF 82 kb)
Supplementary Video 1
Lymphocyte-endothelial interactions in nodal venules of a WBH-treated mouse shown by intravital microscopy. (MOV 2916 kb)
Rights and permissions
About this article
Cite this article
Chen, Q., Fisher, D., Clancy, K. et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol 7, 1299–1308 (2006). https://doi.org/10.1038/ni1406
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1406
This article is cited by
-
Thermal immuno-nanomedicine in cancer
Nature Reviews Clinical Oncology (2023)
-
Intravital three-photon microscopy allows visualization over the entire depth of mouse lymph nodes
Nature Immunology (2022)
-
Transient 40 °C-shock potentiates cytotoxic responses of Vδ2+ γδ T cell via HSP70 upregulation
Cancer Immunology, Immunotherapy (2022)
-
Der Gasteiner Heilstollen und eine mögliche Ansteckungsgefahr im Therapiebereich mit Viren
rheuma plus (2020)
-
Do tumor exosome integrins alone determine organotropic metastasis?
Molecular Biology Reports (2020)