Abstract
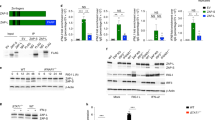
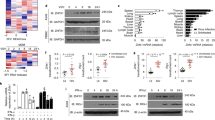
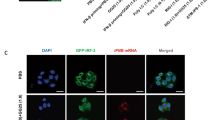
Intracellular detection of RNA virus infection is mediated by the RNA helicase RIG-I, which is recruited to mitochondria by the adaptor protein MAVS and triggers activation of the transcription factors NF-κB, IRF3 and IRF7. Here we demonstrate that virus-induced activation of IRF3 and IRF7 depended on the NF-κB modulator NEMO, which acted 'upstream' of the kinases TBK1 and IKKε. IRF3 phosphorylation, formation of IRF3 dimers and DNA binding, as well as IRF3-dependent gene expression, were abrogated in NEMO-deficient cells. IRF3 phosphorylation and interferon production were restored by ectopic expression of NEMO. Thus, NEMO, like MAVS, acts as an adaptor protein that allows RIG-I to activate both the NF-κB and IRF signaling pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Honda, K., Takaoka, A. & Taniguchi, T. Type I inteferon gene induction by the interferon regulatory factor family of transcription factors. Immunity 25, 349–360 (2006).
Kawai, T. & Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 7, 131–137 (2006).
Meylan, E. & Tschopp, J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol. Cell 22, 561–569 (2006).
Stetson, D.B. & Medzhitov, R. Type I interferons in host defense. Immunity 25, 373–381 (2006).
Pichlmair, A. et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314, 997–1001 (2006).
Hornung, V. et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Maniatis, T. et al. Structure and function of the interferon-β enhanceosome. Cold Spring Harb. Symp. Quant. Biol. 63, 609–620 (1998).
Taniguchi, T., Ogasawara, K., Takaoka, A. & Tanaka, N. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19, 623–655 (2001).
Hiscott, J., Nguyen, T.L., Arguello, M., Nakhaei, P. & Paz, S. Manipulation of the nuclear factor-κB pathway and the innate immune response by viruses. Oncogene 25, 6844–6867 (2006).
van Boxel-Dezaire, A.H., Rani, M.R. & Stark, G.R. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25, 361–372 (2006).
Chen, Z.J. Ubiquitin signalling in the NF-κB pathway. Nat. Cell Biol. 7, 758–765 (2005).
Sharma, S. et al. Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151 (2003).
Fitzgerald, K.A. et al. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 (2003).
Seth, R.B., Sun, L., Ea, C.K. & Chen, Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell 122, 669–682 (2005).
Xu, L.G. et al. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol. Cell 19, 727–740 (2005).
Kawai, T. et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6, 981–988 (2005).
Meylan, E. et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, 1167–1172 (2005).
Pomerantz, J.L. & Baltimore, D. NF-κB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 18, 6694–6704 (1999).
Marie, I., Durbin, J.E. & Levy, D.E. Differential viral induction of distinct interferon-α genes by positive feedback through interferon regulatory factor-7. EMBO J. 17, 6660–6669 (1998).
Zhang, S.Q., Kovalenko, A., Cantarella, G. & Wallach, D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKγ) upon receptor stimulation. Immunity 12, 301–311 (2000).
Servant, M.J. et al. Identification of the minimal phosphoacceptor site required for in vivo activation of interferon regulatory factor 3 in response to virus and double-stranded RNA. J. Biol. Chem. 278, 9441–9447 (2003).
Lin, R., Heylbroeck, C., Pitha, P.M. & Hiscott, J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18, 2986–2996 (1998).
Chariot, A. et al. Association of the adaptor TANK with the IκB kinase (IKK) regulator NEMO connects IKK complexes with IKKε and TBK1 kinases. J. Biol. Chem. 277, 37029–37036 (2002).
Guo, B. & Chang, G. Modulation of the interferon antiviral response by the TBK1/IKKI adaptor protein tank. J. Biol. Chem. 282, 11817–11826 (2007).
Agou, F. et al. The trimerization domain of NEMO is composed of the interacting C-terminal CC2 and LZ coiled-coil subdomains. J. Biol. Chem. 279, 27861–27869 (2004).
Uzel, G. The range of defects associated with nuclear factor κB essential modulator. Curr. Opin. Allergy Clin. Immunol. 5, 513–518 (2005).
Ku, C.L. et al. NEMO mutations in two unrelated boys with severe infections and conical teeth. Pediatrics 115, e615–e619 (2005).
Wu, C.J., Conze, D.B., Li, T., Srinivasula, S.M. & Ashwell, J.D. NEMO is a sensor of Lys 63-linked polyubiquitination and functions in NF-κB activation. Nat. Cell Biol. 8, 398–406 (2006).
Ea, C.K., Deng, L., Xia, Z.P., Pineda, G. & Chen, Z.J. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22, 245–257 (2006).
Rudolph, D. et al. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev. 14, 854–862 (2000).
Makris, C. et al. Female mice heterozygous for IKKγ/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol. Cell 5, 969–979 (2000).
Nomura, F., Kawai, T., Nakanishi, K. & Akira, S. NF-κB activation through IKK-i-dependent I-TRAF/TANK phosphorylation. Genes Cells 5, 191–202 (2000).
Sasai, M. et al. Cutting Edge: NF-κB-activating kinase-associated protein 1 participates in TLR3/Toll-IL-1 homology domain-containing adapter molecule-1-mediated IFN regulatory factor 3 activation. J. Immunol. 174, 27–30 (2005).
Hiscott, J., Lin, R., Nakhaei, P. & Paz, S. MasterCARD: a priceless link to innate immunity. Trends Mol. Med. 12, 53–56 (2006).
Saha, S.K. et al. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 25, 3257–3263 (2006).
Lin, R. et al. Dissociation of a MAVS/IPS-1/VISA/Cardif-IKKε molecular complex from the mitochondrial outer membrane by hepatitis C virus NS3–4A proteolytic cleavage. J. Virol. 80, 6072–6083 (2006).
Nelson, D.L. NEMO, NFκB signaling and incontinentia pigmenti. Curr. Opin. Genet. Dev. 16, 282–288 (2006).
Orange, J.S. et al. The presentation and natural history of immunodeficiency caused by nuclear factor κB essential modulator mutation. J. Allergy Clin. Immunol. 113, 725–733 (2004).
Lin, R. et al. Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20. J. Biol. Chem. 281, 2095–2103 (2006).
Harris, J. et al. Nuclear accumulation of cRel following C-terminal phosphorylation by TBK1/IKKε. J. Immunol. 177, 2527–2535 (2006).
Carter, R.S., Pennington, K.N., Ungurait, B.J. & Ballard, D.W. In vivo identification of inducible phosphoacceptors in the IKKγ/NEMO subunit of human IκB kinase. J. Biol. Chem. 278, 19642–19648 (2003).
Morgenstern, J.P. & Land, H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18, 3587–3596 (1990).
Sato, M. et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity 13, 539–548 (2000).
Li, Z.W. et al. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J. Exp. Med. 189, 1839–1845 (1999).
Li, Q., Estepa, G., Memet, S., Israel, A. & Verma, I.M. Complete lack of NF-κB activity in IKK1 and IKK2 double-deficient mice: additional defect in neurulation. Genes Dev. 14, 1729–1733 (2000).
Zhou, J. & Aiken, C. Nef enhances human immunodeficiency virus type 1 infectivity resulting from intervirion fusion: evidence supporting a role for Nef at the virion envelope. J. Virol. 75, 5851–5859 (2001).
Paz, S. et al. Induction of IRF-3 and IRF-7 phosphorylation following activation of the RIG-I pathway. Cell Mol. Biol. (Noisy-le-grand) 52, 17–28 (2006).
Lin, R., Mamane, Y. & Hiscott, J. Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol. Cell. Biol. 19, 2465–2474 (1999).
tenOever, B.R. et al. Activation of TBK1 and IKKepsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J. Virol. 78, 10636–10649 (2004).
Acknowledgements
We thank M. Schmidt-Supprian, S.C. Sun, S. Yamaoka, G. Sen and T. Dermody for reagents used in this study; D. Goubau and M. Solis for isolation of human primary macrophages; and members of the Molecular Oncology Group at the Lady Davis Institute for discussions. We thank J.D. Ashwell (National Institutes of Health) for plasmids encoding human full-length NEMO, NEMO constructs of amino acids 1–395 or 251–419, and the L329P NEMO substitution mutant; K. Mossman (McMaster University) for Irf3−/− or Irf3−/−Irf9−/− MEFs; M. Karin (University of California, San Diego) for Ikbkb−/−, Ikbkg−/− and wild-type MEFs; I. Verma (The Salk Institute) for MEFs derived from mice lacking IKKα and IKKβ; J. Bell (Ottawa Cancer Centre) for VSV whole virus antisera; and I. Julkunen (National Public Health Institute and University of Helsinki) for SV antisera. Supported by the Cancer Research Society (R.L.), Canadian Institutes of Health Research (R.L. and J.H.), the National Cancer Institute of Canada with support from the Canadian Cancer Society (J.H.), the National Institutes of Health (AI052379 and CA082556 to D.B.W.), Fonds de la Recherche en Santé Quebec (R.L.) and the Canadian Institutes of Health Research (J.H.).
Author information
Authors and Affiliations
Contributions
R.L. designed the research, did experiments, analyzed data, supervised all experiments and wrote the paper; T.Z. did RT-PCR, ELISA, RNA interference and fluorescence microscopy assays; L.Y. did EMSAs, plaque assays and some of the immunoblots; Q.S. did the in vitro kinase assays; J.H. and M.A. wrote the paper together; and D.W.B. provided new reagents and contributed to discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
NEMO deletion mutants affecting activation of NF-κB promoter. (PDF 88 kb)
Supplementary Fig. 2
NEMO deletion mutants affecting cytocolic poly(dA:dT)-mediated activation of ISRE promoter. (PDF 74 kb)
Supplementary Fig. 3
NEMO point mutations affecting activation of NF-κB promoter. (PDF 88 kb)
Supplementary Fig. 4
Model of the role of NEMO in RNA virus–triggered activation of the RIG-I–MAVS signaling pathway. (PDF 57 kb)
Supplementary Table 1
List of the primer sequence for RT-PCR. (PDF 44 kb)
Rights and permissions
About this article
Cite this article
Zhao, T., Yang, L., Sun, Q. et al. The NEMO adaptor bridges the nuclear factor-κB and interferon regulatory factor signaling pathways. Nat Immunol 8, 592–600 (2007). https://doi.org/10.1038/ni1465
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1465
This article is cited by
-
LUBAC is required for RIG-I sensing of RNA viruses
Cell Death & Differentiation (2024)
-
Variations in the NSP4 gene of the type 2 porcine reproductive and respiratory syndrome virus isolated in China from 1996 to 2021
Virus Genes (2023)
-
Early innate immune response triggered by the human respiratory syncytial virus and its regulation by ubiquitination/deubiquitination processes
Journal of Biomedical Science (2022)
-
TRIM41 is required to innate antiviral response by polyubiquitinating BCL10 and recruiting NEMO
Signal Transduction and Targeted Therapy (2021)
-
The c-Rel transcription factor limits early interferon and neuroinflammatory responses to prevent herpes simplex encephalitis onset in mice
Scientific Reports (2021)