Abstract
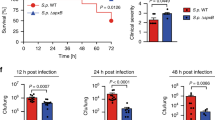
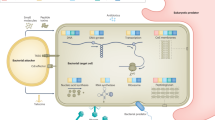
The evolution of the host-pathogen relationship comprises a series of invasive-defensive tactics elicited by both participants. The stereotype is that the antimicrobial immune response requires multistep processes. Little is known about the primordial immunosurveillance system, which probably has components that directly link sensors and effectors. Here we found that the respiratory proteins of both the horseshoe crab and human were directly activated by microbial proteases and were enhanced by pathogen-associated molecular patterns, resulting in the production of more reactive oxygen species. Hemolytic virulent pathogens, which produce proteases as invasive factors, are more susceptible to this killing mechanism. This 'shortcut' antimicrobial strategy represents a fundamental and universal mode of immunosurveillance, which has been in existence since before the split of protostomes and deuterostomes and still persists today.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kuehn, M.J. & Kesty, N.C. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19, 2645–2655 (2005).
Winzer, K. & Williams, P. Quorum sensing and the regulation of virulence gene expression in pathogenic bacteria. Int. J. Med. Microbiol. 291, 131–143 (2001).
Costerton, J.W., Stewart, P.S. & Greenberg, E.P. Bacterial biofilm: a common cause of persistent infections. Science 284, 1318–1322 (1999).
Murphy, P.M. Viral exploitation and subversion of the immune system through chemokine mimicry. Nat. Immunol. 2, 116–122 (2001).
Ganz, T. & Lehrer, R.I. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 10, 41–44 (1998).
Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2, 820–832 (2004).
Muller-Eberhard, H.J. Molecular organization and function of the complement system. Annu. Rev. Biochem. 57, 321–347 (1988).
Medzhitov, R. & Janeway, C. Jr. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 173, 89–97 (2000).
Iwanaga, S. The molecular basis of innate immunity in the horseshoe crab. Curr. Opin. Immunol. 14, 87–95 (2002).
Kawano, T., Pinontoan, R., Hosoya, H. & Muto, S. Monoamine-dependent production of reactive oxygen species catalyzed by pseudoperoxidase activity of human hemoglobin. Biosci. Biotechnol. Biochem. 66, 1224–1232 (2002).
Twenhofel, W.H. & Shrock, R.R. Invertebrate Paleontology (eds. Twenhofel, W.H. & Shrock, R.R.) 406–477 (McGraw-Hill, New York, 1935).
Ding, J.L. et al. Spatial and temporal coordination of expression of immune response genes during Pseudomonas aeruginosa infection of horseshoe crab, Carcinoscorpius rotundicauda. Genes Immun. 6, 557–574 (2005).
Decker, H., Ryan, M., Jaenicke, E. & Terwilliger, N. SDS-induced phenol-oxidase activity of hemocyanins from Limulus polyphemus, Eurypelma californicum, and Cancer magister. J. Biol. Chem. 276, 17796–17799 (2001).
Bolton, J.L., Trush, M.A., Penning, T.M., Dryhurst, G. & Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 13, 135–160 (2000).
Nagai, T. & Kawabata, S. A link between blood coagulation and prophenol oxidase activation in arthropod host defense. J. Biol. Chem. 275, 29264–29267 (2000).
Nagai, T., Osaki, T. & Kawabata, S. Functional conversion of hemocyanin to phenoloxidase by horseshoe crab antimicrobial peptides. J. Biol. Chem. 276, 27166–27170 (2001).
Cerenius, L. & Soderhall, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 198, 116–126 (2004).
Alayash, A.I. Hemoglobin-based blood substitutes: oxygen carriers, pressor agents, or oxidants? Nat. Biotechnol. 17, 545–549 (1999).
Fee, J.A. in Oxygen and Oxyradicals in Chemistry and Biology (eds. Rodgers, M.A.J. & Powers, E.L.) 205–239 (Academic, New York, 1981).
Wentworth, A.D., Jones, L.H., Wentworth, P., Janda, K.D. & Lerner, R.A. Antibodies have the intrinsic capacity to destroy antigens. Proc. Natl. Acad. Sci. USA 97, 10930–10935 (2000).
Bunn, H.F. & Forget, B.G. Hemoglobin: Molecular Genetic and Clinical Aspects (eds. Bunn, H.F. & Forget, B.G.) 634–662 (W.B. Saunders, Philadelphia, 1986).
Skaar, E.P., Humayun, M., Bae, T., DeBord, K.L. & Schneewind, O. Iron-source preference of Staphylococcus aureus infections. Science 305, 1626–1628 (2004).
Heck, L.W. et al. Degradation of IgA proteins by Pseudomonas aeruginosa elastase. J. Immunol. 144, 2253–2257 (1990).
Wretlind, B. & Pavlovskis, O.R. Pseudomonas aeruginosa elastase and its role in pseudomonas infections. Rev. Infect. Dis. 5, 998–1004 (1983).
Chan, P.F. & Foster, S.J. Role of SarA in virulence determination production and environmental signal transduction in Staphylococcus aureus. J. Bacteriol. 180, 6232–6241 (1998).
Zhu, Y., Thangamani, S., Ho, B. & Ding, J.L. The ancient origin of the complement system. EMBO J. 24, 382–394 (2005).
Ng, P.M., Jin, Z., Tan, S.S., Ho, B. & Ding, J.L. C-reactive protein: a predominant LPS-binding acute phase protein responsive to pseudomonas infection. J. Endotoxin Res. 10, 163–174 (2004).
Decker, H. & Tuczek, F. Tyrosinase/catecholoxidase activity of hemocyanins: structural basis and molecular mechanism. Trends Biochem. Sci. 25, 392–397 (2000).
Toder, D.S., Ferrell, S.J., Nezezon, J.L., Rust, L. & Iglewski, B.H. lasA and lasB genes of Pseudomonas aeruginosa: analysis of transcription and gene product activity. Infect. Immun. 62, 1320–1327 (1994).
Nellaiappan, K. & Sugumaran, M. On the presence of prophenoloxidase in the hemolymph of the horseshoe crab, Limulus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 113, 163–168 (1996).
Lee, S.Y., Lee, B.L. & Soderhall, K. Processing of an antibacterial peptide from hemocyanin of the freshwater crayfish Pacifastacus leniusculus. J. Biol. Chem. 278, 7927–7933 (2003).
Karlsson, A., Saravia-Otten, P., Tegmark, K., Morfeldt, E. & Arvidson, S. Decreased amounts of cell wall-associated protein A and fibronectin-binding proteins in Staphylococcus aureus sarA mutants due to up-regulation of extracellular proteases. Infect. Immun. 69, 4742–4748 (2001).
Dowd, P.F. Relative inhibition of insect phenoloxidase by cyclic fungal metabolites from insect and plant pathogens. Nat. Toxins 7, 337–341 (1999).
Ariki, S. et al. A serine protease zymogen functions as a pattern-recognition receptor for lipopolysaccharides. Proc. Natl. Acad. Sci. USA 101, 953–958 (2004).
Zhu, Y., Ng, P.M.L., Wang, L., Ho, B. & Ding, J.L. Diversity in lectins enables immune recognition and differentiation of wide spectrum of pathogens. Int. Immunol. 18, 1671–1680 (2006).
Kodati, V.R. & Lafleur, M. Comparison between orientational and conformational orders in fluid lipid bilayers. Biophys. J. 64, 163–170 (1993).
Osawa, T. & Kato, Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann. NY Acad. Sci. 1043, 440–451 (2005).
Hampton, M.B., Kettle, A.J. & Winterbourn, C.C. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92, 3007–3017 (1998).
Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Ding, J.L., Navas, M.A.A. III & Ho, B. Two forms of factor C from the amoebocytes of Carcinoscorpius rotundicauda: purification and characterization. Biochim. Biophys. Acta 1202, 149–156 (1993).
Wang, J., Ho, B. & Ding, J.L. Functional expression of full length Limulus factor C in stably transformed Sf9 cells. Biotechnol. Lett. 23, 71–76 (2001).
Brenowitz, M., Bonaventura, C., Bonaventura, J. & Gianazza, E. Subunit composition of high molecular weight oligomer: Limulus polyphemus hemocyanin. Arch. Biochem. Biophys. 210, 748–761 (1981).
Heinz, D., Margaret, R., Elmar, J. & Nora, T. SDS-induced phenoloxidase activity of hemocyanin from Limulus polyphemus, Eurupelma californicum, and Cancer magister. J. Biol. Chem. 276, 17796–17799 (2001).
Jiang, H., Wang, Y. & Kanost, M.R. Pro-phenol oxidase activating proteinase from an insect, Manduca sexta: a bacteria-inducible protein similar to Drosophila easter. Proc. Natl. Acad. Sci. USA 95, 12220–12225 (1998).
Acknowledgements
We thank B.H. Iglewski (University of Rochester School of Medicine and Dentistry) for P. aeruginosa strains PAO-Iglewski and PAO-B1A1; S. Arvidson (Karolinska University, Sweden) for S. aureus strains PC1839 and AK3; L. Zhang (Institute of Molecular and Cell Biology, Singapore) for plasmid pDSK-GFP; A. Cheung (Dartmouth Medical School) for plasmid pALC1420; H.C. Ng for bacterial isolation and technical support; S. Leptihn for suggestions on bacterial identification by 16S rRNA sequencing; and B. Halliwell, A. Le Saux and P.M.L. Ng for critical reading of the manuscript. Supported by the Agency for Science Technology and Research (Biomedical Research Council), the Academic Research Fund of the Ministry of Education, and the Faculty Research Committee of the National University of Singapore.
Author information
Authors and Affiliations
Contributions
N.J. designed and did the experiments and prepared the manuscript; N.S.T. provided some suggestions, helped with real-time fluorescence imaging and manuscript preparation; B.H. provided expertise in microbiology, designed and did some of the experiments and participated in manuscript preparation; J.L.D. conceived the ideas, designed the biochemical, cell and molecular biology experiments, and supervised the studies and preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11, Methods and Text (PDF 14224 kb)
Supplementary Video 1
The real-time imaging of the bacterial clearance elicited by PO-catalyzed production of quinone using PAO-Iglewski, the wild type Pseudomonas aeruginosa strain which produces extracellular protease. (MOV 993 kb)
Supplementary Video 2
The real-time imaging of the bacterial clearance elicited by PO-catalyzed production of quinone using PAO-B1A1, the Pseudomonas aeruginosa mutant strain which is protease-deficient. (MOV 1183 kb)
Supplementary Video 3
The real-time imaging of the bacterial clearance elicited by PO-catalyzed production of quinone using PAO-Iglewski in the presence of phenylthiourea, a specific inhibitor of the phenoloxidase. (MOV 803 kb)
Rights and permissions
About this article
Cite this article
Jiang, N., Tan, N., Ho, B. et al. Respiratory protein–generated reactive oxygen species as an antimicrobial strategy. Nat Immunol 8, 1114–1122 (2007). https://doi.org/10.1038/ni1501
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1501
This article is cited by
-
The effect of dietary supplementation of proteases on growth, digestive enzymes, oxidative stress, and intestinal morphology in fishes – A review
Aquaculture International (2023)
-
Polyinosinic:polycytidylic acid in vivo enhances Chinook salmon (Oncorhynchus tshawytscha) immunity and alters the fish metabolome
Aquaculture International (2020)
-
Transcriptomic analysis of immune response to bacterial lipopolysaccharide in zebra finch (Taeniopygia guttata)
BMC Genomics (2019)
-
Antibacterial Natural Peptide Fractions from Indian Ganoderma lucidum
International Journal of Peptide Research and Therapeutics (2018)
-
Phagocytosis and oxycytosis: two arms of human innate immunity
Immunologic Research (2018)