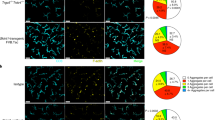
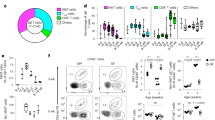
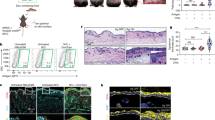
Abstract
The self-encoded ligands MICA (human) and Rae-1 (mouse) for the cytotoxic lymphocyte activating receptor NKG2D are highly expressed in carcinomas and inflammatory lesions and have been linked to immunosurveillance and graft rejection. However, whether NKG2D ligands have an intrinsic ability to acutely regulate tissue-associated immune compartments is not known. Here we show that epidermis-specific upregulation of Rae-1 induced rapid, coincident and reversible changes in the organization of tissue-resident Vγ5Vδ1 TCRγδ+ intraepithelial T cells and Langerhans cells, swiftly followed by epithelial infiltration by unconventional αβ T cells. Whereas local Vγ5Vδ1+ T cells limited carcinogenesis, Langerhans cells unexpectedly promoted it. These results provide unique insight into the early phases of tissue immunosurveillance and indicate that acute changes in NKG2D ligands may alone initiate a rapid, multifaceted immunosurveillance response in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Smyth, M.J., Dunn, G.P. & Schreiber, R.D. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 90, 1–50 (2006).
Qin, Z. & Blankenstein, T. A cancer immunosurveillance controversy. Nat Immunol 5, 3–4 (2004).
Cui, J. et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 278, 1623–1626 (1997).
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997).
Girardi, M. et al. Regulation of cutaneous malignancy by γδ T cells. Science 294, 605–609 (2001).
Girardi, M. et al. The distinct contributions of murine T cell receptor (TCR) γδ+ and TCR αβ+ T cells to different stages of chemically induced skin cancer. J. Exp. Med. 198, 747–755 (2003).
Wilhelm, M. et al. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood 102, 200–206 (2003).
Dieli, F. et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 67, 7450–7457 (2007).
Bauer, S. et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 (1999).
Diefenbach, A., Jamieson, A.M., Liu, S.D., Shastri, N. & Raulet, D.H. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 1, 119–126 (2000).
Cerwenka, A. et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 12, 721–727 (2000).
Carayannopoulos, L.N., Naidenko, O.V., Fremont, D.H. & Yokoyama, W.M. Cutting edge: murine UL16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D. J. Immunol. 169, 4079–4083 (2002).
Groh, V. et al. Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MICA and MICB. Proc. Natl. Acad. Sci. USA 96, 6879–6884 (1999).
Gasser, S., Orsulic, S., Brown, E.J. & Raulet, D.H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436, 1186–1190 (2005).
Rolle, A. et al. Effects of human cytomegalovirus infection on ligands for the activating NKG2D receptor of NK cells: up-regulation of UL16-binding protein (ULBP)1 and ULBP2 is counteracted by the viral UL16 protein. J. Immunol. 171, 902–908 (2003).
Zou, Y., Stastny, P., Susal, C., Dohler, B. & Opelz, G. Antibodies against MICA antigens and kidney-transplant rejection. N. Engl. J. Med. 357, 1293–1300 (2007).
Ogasawara, K. et al. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity 20, 757–767 (2004).
Groh, V., Bruhl, A., El-Gabalawy, H., Nelson, J.L. & Spies, T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 100, 9452–9457 (2003).
Smyth, M.J. et al. NKG2D function protects the host from tumor initiation. J. Exp. Med. 202, 583–588 (2005).
Groh, V., Wu, J., Yee, C. & Spies, T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419, 734–738 (2002).
Dunn, G.P., Old, L.J. & Schreiber, R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22, 329–360 (2004).
Wiemann, K. et al. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J. Immunol. 175, 720–729 (2005).
Oppenheim, D.E. et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat. Immunol. 6, 928–937 (2005).
Sporri, R. & Reis e Sousa, C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat. Immunol. 6, 163–170 (2005).
Mantovani, A., Bottazzi, B., Colotta, F., Sozzani, S. & Ruco, L. The origin and function of tumor-associated macrophages. Immunol. Today 13, 265–270 (1992).
Roberts, S.J. et al. Characterizing tumor-promoting T cells in chemically induced cutaneous carcinogenesis. Proc. Natl. Acad. Sci. USA 104, 6770–6775 (2007).
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Born, W. et al. Immunoregulatory functions of γδ T cells. Adv. Immunol. 71, 77–144 (1999).
Girardi, M. et al. Resident skin-specific γδ T cells provide local, nonredundant regulation of cutaneous inflammation. J. Exp. Med. 195, 855–867 (2002).
Kaplan, D.H., Jenison, M.C., Saeland, S., Shlomchik, W.D. & Shlomchik, M.J. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity 23, 611–620 (2005).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Graham, D.B. et al. Vav1 controls DAP10-mediated natural cytotoxicity by regulating actin and microtubule dynamics. J. Immunol. 177, 2349–2355 (2006).
Nishibu, A. et al. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J. Invest. Dermatol. 126, 787–796 (2006).
Jameson, J.M., Cauvi, G., Witherden, D.A. & Havran, W.L. A keratinocyte-responsive γδ TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J. Immunol. 172, 3573–3579 (2004).
Bendelac, A., Savage, P.B. & Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336 (2007).
Godfrey, D.I., MacDonald, H.R., Kronenberg, M., Smyth, M.J. & Van Kaer, L. NKT cells: what's in a name? Nat. Rev. Immunol. 4, 231–237 (2004).
Miller, S.J. et al. Mouse skin is particularly susceptible to tumor initiation during early anagen of the hair cycle: possible involvement of hair follicle stem cells. J. Invest. Dermatol. 101, 591–594 (1993).
Trempus, C.S. et al. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 67, 4173–4181 (2007).
Mallick-Wood, C.A. et al. Conservation of T cell receptor conformation in epidermal γδ cells with disrupted primary Vγ gene usage. Science 279, 1729–1733 (1998).
Willimsky, G. & Blankenstein, T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature 437, 141–146 (2005).
Schreiber, R.D., Old, L.J., Hayday, A.C. & Smyth, M.J. Response to 'A cancer immunosurveillance controversy'. Nat. Immunol. 5, 4–5 (2004).
Bonish, B. et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-γ production by NK-T cells. J. Immunol. 165, 4076–4085 (2000).
Cameron, A.L., Kirby, B., Fei, W. & Griffiths, C.E. Natural killer and natural killer-T cells in psoriasis. Arch. Dermatol. Res. 294, 363–369 (2002).
Moodycliffe, A.M., Nghiem, D., Clydesdale, G. & Ullrich, S.E. Immune suppression and skin cancer development: regulation by NKT cells. Nat. Immunol. 1, 521–525 (2000).
Ambrosino, E. et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J. Immunol. 179, 5126–5136 (2007).
Terabe, M. et al. A nonclassical non-Vα14Jα18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J. Exp. Med. 202, 1627–1633 (2005).
Stephens, H.A. MICA and MICB genes: can the enigma of their polymorphism be resolved? Trends Immunol 22, 378–385 (2001).
Jinushi, M., Hodi, F.S. & Dranoff, G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc. Natl. Acad. Sci. USA 103, 9190–9195 (2006).
Grossman, Z. & Paul, W.E. Adaptive cellular interactions in the immune system: the tunable activation threshold and the significance of subthreshold responses. Proc. Natl. Acad. Sci. USA 89, 10365–10369 (1992).
Lewis, J.M. et al. Selection of the cutaneous intraepithelial γδ+ T cell repertoire by a thymic stromal determinant. Nat. Immunol. 7, 843–850 (2006).
Acknowledgements
We thank R. Tigelaar, T. Silberzahn, J. Dyson, D. Oppenheim, M. Shlomchik and R. Montgomery for reagents, help and discussions. Supported by the National Cancer Institute (R01-CA102703 and P50-CA121974 to M.G.) and the Wellcome Trust (071534 to A.C.H. and J.S.).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Methods (PDF 1349 kb)
Supplementary Video 1
Interdigitating network of LC and DETC in resting murine epidermis. Three-dimensional analysis of the spatial relation between MHCII+ LC (green) and TCRγδ+ DETC (red) in the epidermis. Confocal microscopy image of epidermal sheet from wild-type FVB mouse was interpreted in 3D using Imaris software (Bitplane). (MOV 10861 kb)
Supplementary Video 2
Juxtaposed LC and DETC in the epidermis following local upregulation of Rae-1. Confocal microscopy image of epidermal sheet from a BiTg mouse after 72h on dox. MHCII+ LC (green); TCRγδ+ DETC (red). 3D rendering of confocal optical sections was prepared using Imaris software (Bitplane). (MOV 7211 kb)
Rights and permissions
About this article
Cite this article
Strid, J., Roberts, S., Filler, R. et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol 9, 146–154 (2008). https://doi.org/10.1038/ni1556
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1556
This article is cited by
-
Emerging hallmark of gliomas microenvironment in evading immunity: a basic concept
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2023)
-
Local γδ T cells: translating promise to practice in cancer immunotherapy
British Journal of Cancer (2023)
-
Langerhans cells shape postnatal oral homeostasis in a mechanical-force-dependent but microbiota and IL17-independent manner
Nature Communications (2023)
-
γδ T Cells and Allergic Diseases
Clinical Reviews in Allergy & Immunology (2023)
-
Normality sensing licenses local T cells for innate-like tissue surveillance
Nature Immunology (2022)