Abstract
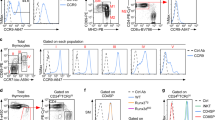
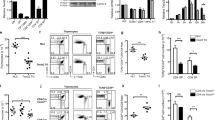
The migration patterns of naive and activated T cells are associated with the expression of distinct sets of chemokine receptors, but the molecular basis for this regulation is unknown. Here we identify Krupple-like factor 2 (KLF2) as a key transcriptional factor needed to prevent naive T cells from expressing inflammatory chemokine receptors and acquiring the migration patterns of activated T cells. Lineage-specific deletion of KLF2 resulted in fewer naive T cells in the blood and secondary lymphoid organs, whereas it expanded naive T cell numbers in nonlymphoid tissues; these effects were associated with altered expression of inflammatory chemokine receptors on naive T cells. KLF2 repressed the expression of several chemokine receptors, including CCR3 and CCR5. We thus conclude that KLF2 maintains proper T cell migration patterns by linking T cell movement and transcriptional regulation of chemokine receptor expression patterns.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dang, D.T., Pevsner, J. & Yang, V.W. The biology of the mammalian Kruppel-like family of transcription factors. Int. J. Biochem. Cell Biol. 32, 1103–1121 (2000).
Kaczynski, J., Cook, T. & Urrutia, R. Sp1- and Kruppel-like transcription factors. Genome Biol. 4, 206.1–206.8 (2003).
Schober, S.L. et al. Expression of the transcription factor lung Kruppel-like factor is regulated by cytokines and correlates with survival of memory T cells in vitro and in vivo. J. Immunol. 163, 3662–3667 (1999).
Kuo, C.T., Veselits, M.L. & Leiden, J.M. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science 277, 1986–1990 (1997).
Kuo, C.T. et al. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 11, 2996–3006 (1997).
Wani, M.A., Means, R.T. Jr. & Lingrel, J.B. Loss of LKLF function results in embryonic lethality in mice. Transgenic Res. 7, 229–238 (1998).
Lee, J.S. et al. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev. Cell 11, 845–857 (2006).
Buckley, A.F., Kuo, C.T. & Leiden, J.M. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc–dependent pathway. Nat. Immunol. 2, 698–704 (2001).
Carlson, C.M. et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 442, 299–302 (2006).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Allende, M.L., Dreier, J.L., Mandala, S. & Proia, R.L. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 279, 15396–15401 (2004).
Teresa Sanchez, T.H. Structural and functional characteristics of S1P receptors. J. Cell. Biochem. 92, 913–922 (2004).
Chiba, K., Matsuyuki, H., Maeda, Y. & Sugahara, K. Role of sphingosine 1-phosphate receptor type 1 in lymphocyte egress from secondary lymphoid tissues and thymus. Cell. Mol. Immunol. 3, 11–19 (2006).
Stadtfeld, M. & Graf, T. Assessing the role of hematopoietic plasticity for endothelial and hepatocyte development by non-invasive lineage tracing. Development 132, 203–213 (2005).
Kuo, C.T., Veselits, M.L. & Leiden, J.M. LKLF and FasL expression: correctiona and clarification. Science 278, 788–789 (1997).
Lee, M.-J. et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279, 1552–1555 (1998).
Liu, C.H. et al. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell 10, 1179–1190 (1999).
Graeler, M. & Goetzl, E.J. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 16, 1874–1878 (2002).
Pappu, R. et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298 (2007).
Chiba, K. et al. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J. Immunol. 160, 5037–5044 (1998).
Graler, M.H. & Goetzl, E.J. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 18, 551–553 (2004).
Luster, A.D., Alon, R. & von Andrian, U.H. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 6, 1182–1190 (2005).
Cyster, J.G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 (2005).
von Andrian, U.H. & Mempel, T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3, 867–878 (2003).
Bai, A., Hu, H., Yeung, M. & Chen, J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J. Immunol. 178, 7632–7639 (2007).
Liu, R., Zhao, X., Gurney, T. & Landau, N. Functional analysis of the proximal CCR5 promoter. AIDS Res. Hum. Retroviruses 14, 1509–1519 (1998).
Guignard, F., Combadiere, C., Tiffany, H.L. & Murphy, P.M. Gene organization and promoter function for CC chemokine receptor 5 (CCR5). J. Immunol. 160, 985–992 (1998).
Scotet, E., Schroeder, S. & Lanzavecchia, A. Molecular regulation of CC-chemokine receptor 3 expression in human T helper 2 cells. Blood 98, 2568–2570 (2001).
Gonzalo, J.-A. et al. Mouse eotaxin expression parallels eosinophil accumulation during lung allergic inflammation but it is not restricted to a Th2-type response. Immunity 4, 1–14 (1996).
Heath, H. et al. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J. Clin. Invest. 99, 178–184 (1997).
Apolinario, A. et al. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am. J. Gastroenterol. 97, 2861–2870 (2002).
Murai, M. et al. Active participation of CCR5+CD8+ T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J. Clin. Invest. 104, 49–57 (1999).
Ajuebor, M.N., Hogaboam, C.M., Le, T., Proudfoot, A.E. & Swain, M.G. CCL3/MIP-1α is pro-inflammatory in murine T cell-mediated hepatitis by recruiting CCR1-expressing CD4+ T cells to the liver. Eur. J. Immunol. 34, 2907–2918 (2004).
von Andrian, U.H. & Mackay, C.R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343, 1020–1034 (2000).
Rot, A. & von Andrian, U.H. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 22, 891–928 (2004).
Ebert, L.M., Schaerli, P. & Moser, B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol. Immunol. 42, 799–809 (2005).
Rosen, S.D. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 22, 129–156 (2004).
Daugherty, B. et al. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J. Exp. Med. 183, 2349–2354 (1996).
Uguccioni, M. et al. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J. Clin. Invest. 100, 1137–1143 (1997).
Ochi, H. et al. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J. Exp. Med. 190, 267–280 (1999).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Lefrancois, L. Development, trafficking, and function of memory T-cell subsets. Immunol. Rev. 211, 93–103 (2006).
Endrizzi, B.T. & Jameson, S.C. Differential role for IL-7 in inducing lung Kruppel-like factor (Kruppel-like factor 2) expression by naive versus activated T cells. Int. Immunol. 15, 1341–1348 (2003).
Lee, P.P. et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15, 763–774 (2001).
Acknowledgements
We thank J. Cyster and M. Zachariah for assistance with anti-S1P1 staining and for comments on the manuscript; T. Graf (Albert Einstein College of Medicine) for the use of Vav-Cre–transgenic mice; and G. Koretzky, J. Maltzman and B. Kleaveland for insights. The plasmid pcDNA-HA-KLF2 was a gift from laboratory of L.H. Glimcher (Harvard School of Public Health).
Author information
Authors and Affiliations
Contributions
E.S. designed and did experiments and wrote the manuscript; Z.Z., J.S.L. and T.W. designed and did experiments; and M.L.K. designed experiments and wrote the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–2 and Table 1 (PDF 389 kb)
Rights and permissions
About this article
Cite this article
Sebzda, E., Zou, Z., Lee, J. et al. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol 9, 292–300 (2008). https://doi.org/10.1038/ni1565
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1565
This article is cited by
-
Single-cell analysis reveals differences among iNKT cells colonizing peripheral organs and identifies Klf2 as a key gene for iNKT emigration
Cell Discovery (2022)
-
S1PR1 induces metabolic reprogramming of ceramide in vascular endothelial cells, affecting hepatocellular carcinoma angiogenesis and progression
Cell Death & Disease (2022)
-
The pharmaceutical solvent N-methyl-2-pyrollidone (NMP) attenuates inflammation through Krüppel-like factor 2 activation to reduce atherogenesis
Scientific Reports (2020)
-
Chemokine mediated signalling within arteries promotes vascular smooth muscle cell recruitment
Communications Biology (2020)
-
Aquaporin 4 inhibition alters chemokine receptor expression and T cell trafficking
Scientific Reports (2019)