Abstract
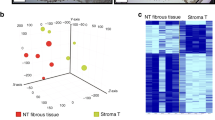
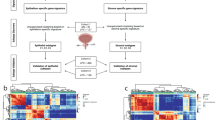
To better understand the relationship between tumor-host interactions and the efficacy of chemotherapy, we have developed an analytical approach to quantify several biological processes observed in gene expression data sets. We tested the approach on tumor biopsies from individuals with estrogen receptor–negative breast cancer treated with chemotherapy. We report that increased stromal gene expression predicts resistance to preoperative chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide (FEC) in subjects in the EORTC 10994/BIG 00-01 trial. The predictive value of the stromal signature was successfully validated in two independent cohorts of subjects who received chemotherapy but not in an untreated control group, indicating that the signature is predictive rather than prognostic. The genes in the signature are expressed in reactive stroma, according to reanalysis of data from microdissected breast tumor samples. These findings identify a previously undescribed resistance mechanism to FEC treatment and suggest that antistromal agents may offer new ways to overcome resistance to chemotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others

References
Colleoni, M. et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin. Cancer Res. 10, 6622–6628 (2004).
Guarneri, V. et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J. Clin. Oncol. 24, 1037–1044 (2006).
Fisher, E.R. et al. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer 95, 681–695 (2002).
Chang, J.C. et al. Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet 362, 362–369 (2003).
Gianni, L. et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J. Clin. Oncol. 23, 7265–7277 (2005).
Hess, K.R. et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin and cyclophosphamide in breast cancer. J. Clin. Oncol. 24, 4236–4244 (2006).
Thuerigen, O. et al. Gene expression signature predicting pathologic complete response with gemcitabine, epirubicin and docetaxel in primary breast cancer. J. Clin. Oncol. 24, 1839–1845 (2006).
Hannemann, J. et al. Changes in gene expression associated with response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 23, 3331–3342 (2005).
Potti, A. et al. Genomic signatures to guide the use of chemotherapeutics. Nat. Med. 12, 1294–1300 (2006).
Bonnefoi, H. et al. Validation of gene signatures that predict the response of breast cancer to neoadjuvant chemotherapy: a substudy of the EORTC 10994/BIG 00–01 clinical trial. Lancet Oncol. 8, 1071–1078 (2007).
Farmer, P. et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene 24, 4660–4671 (2005).
Perou, C.M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Sorlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 98, 10869–10874 (2001).
Sotiriou, C. et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J. Natl. Cancer Inst. 98, 262–272 (2006).
van de Vijver, M.J. et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 347, 1999–2009 (2002).
Bild, A.H., Potti, A. & Nevins, J.R. Linking oncogenic pathways with therapeutic opportunities. Nat. Rev. Cancer 6, 735–741 (2006).
Hu, Z. et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics 7, 96 (2006).
Boersma, B.J. et al. A stromal gene signature associated with inflammatory breast cancer. Int. J. Cancer 122, 1324–1332 (2008).
Dontu, G. et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 17, 1253–1270 (2003).
Mani, S.A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Li, X. et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Natl. Cancer Inst. 100, 672–679 (2008).
West, R.B. et al. Determination of stromal signatures in breast carcinoma. PLoS Biol. 3, e187 (2005).
Wirapati, P. et al. Meta-analysis of gene-expression profiles in breast cancer: toward a unified understanding of breast cancer sub-typing and prognosis signatures. Breast Cancer Res. 10, R65 (2008).
Qiu, W. et al. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat. Genet. 40, 650–655 (2008).
Finak, G. et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 14, 518–527 (2008).
Weaver, V.M. et al. β4 integrin–dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2, 205–216 (2002).
Misra, S., Ghatak, S. & Toole, B.P. Regulation of MDR1 expression and drug resistance by a positive feedback loop involving hyaluronan, phosphoinositide 3-kinase, and ErbB2. J. Biol. Chem. 280, 20310–20315 (2005).
Damiano, J.S., Cress, A.E., Hazlehurst, L.A., Shtil, A.A. & Dalton, W.S. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood 93, 1658–1667 (1999).
Hazlehurst, L.A. et al. Reduction in drug-induced DNA double-strand breaks associated with β1 integrin–mediated adhesion correlates with drug resistance in U937 cells. Blood 98, 1897–1903 (2001).
McAllister, S.S. et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell 133, 994–1005 (2008).
Hedges, L. & Olkin, I. Statistical Methods for Meta-Analysis. 39 (Academic Press, London, 1985).
Westfall, P. & Young, S. Resampling-Based Multiple Testing: Examples And Methods For P-Values Adjustment. Ch.2 (Wiley, New York, 1993).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. [Ser B] 57, 289–300 (1995).
R Development Core Team. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, 2005).
International Union Against Cancer (UICC). TNM Classification of Malignant Tumours 5th edn. (eds. Sobin, L.H. & Wittekind, C.) (Wiley, New York, 1997).
Finak, G. et al. Gene expression signatures of morphologically normal breast tissue identify basal-like tumors. Breast Cancer Res. 8, R58 (2006).
Acknowledgements
We thank the subjects, doctors and nurses involved in the EORTC 10994/BIG01 study for their generous participation. We thank the staff of the EORTC data center (M. de Vos, S. Lejeune, I. Delmotte and M. Karina) and the technician in the Iggo laboratory (A.-L. Nicoulaz) for assistance with data management and sample processing. We thank I. Xenarios, V. Praz and T. Sengstag for support with bioinformatics. We thank H. Chebab (Pathology Institute, University Lausanne) for supplying human colon tumors. We thank M. Zahn for critical reading of the manuscript. We thank the University of Lausanne DNA array facility and the Swiss Institute for Bioinformatics Vital-IT project for infrastructure support. We thank the Fondation Medic, Fondation Widmer, Oncosuisse, Swiss National Science Foundation and Swiss National Center for Competence in Research (NCCR) Molecular Oncology, EORTC Translational Research Fund, Swedish Cancer Society, King Gustav the Fifth Jubilee Fund and Swedish Research Council for financial support.
Author information
Authors and Affiliations
Contributions
J.B., F.B., E. Blot, H.B., D.C., M.C., J.J., G.M., T.P., M.P. and E. Brain supplied tumor tissues and collected the clinical follow-up data. V.B. did the central pathology review. R.I. supervised the laboratory experiments. S.A. performed laboratory experiments. P.F., P.W. and M.D. developed the statistical models. P.F., M.D. and R.I. analyzed the data. J.B. performed additional statistical analyses. P.A. and M.A. performed and supervised additional laboratory experiments. H.B., R.I., P.F. and M.D. designed the study. P.F., M.D., H.B., P.A., J.B., M.A., D.C. and R.I. wrote the report. All investigators contributed to and reviewed the final report.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Table 1, Supplementary Figs. 1–7 and Supplementary Methods (PDF 1286 kb)
Rights and permissions
About this article
Cite this article
Farmer, P., Bonnefoi, H., Anderle, P. et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med 15, 68–74 (2009). https://doi.org/10.1038/nm.1908
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1908
This article is cited by
-
Digital image analysis and machine learning-assisted prediction of neoadjuvant chemotherapy response in triple-negative breast cancer
Breast Cancer Research (2024)
-
Deciphering cellular plasticity in pancreatic cancer for effective treatments
Cancer and Metastasis Reviews (2024)
-
Patient-derived scaffolds representing breast cancer microenvironments influence chemotherapy responses in adapted cancer cells consistent with clinical features
Journal of Translational Medicine (2023)
-
Tumor collagens predict genetic features and patient outcomes
npj Genomic Medicine (2023)
-
Mesenchymal stromal cells confer breast cancer doxorubicin resistance by producing hyaluronan
Oncogene (2023)