Abstract
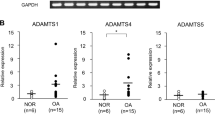
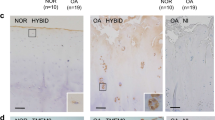
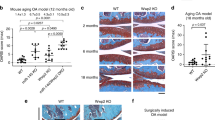
Aggrecan cleavage by a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 5 (ADAMTS-5) is crucial for the breakdown of cartilage matrix during osteoarthritis1,2, a degenerative joint disease that leads to the progressive destruction of articular structures. The mechanisms of ADAMTS-5 activation and their links to the pathogenesis of osteoarthritis remain poorly understood, but syndecans have been shown to be involved in the activation of ADAMTS-4 (ref. 3). Here we show that syndecan-4 is specifically induced in type X collagen–producing chondrocytes both in human osteoarthritis and in murine models of the disease. The loss of syndecan-4 in genetically modified mice and intra-articular injections of syndecan-4–specific antibodies into wild-type mice protect from proteoglycan loss and thereby prevent osteoarthritic cartilage damage in a surgically induced model of osteoarthritis. The occurrence of less severe osteoarthritis-like cartilage destruction in both syndecan-4–deficient mice and syndecan-4–specific antibody–treated wild-type mice results from a marked decrease in ADAMTS-5 activity. Syndecan-4 controls the activation of ADAMTS-5 through direct interaction with the protease and through regulating mitogen-activated protein kinase (MAPK)-dependent synthesis of matrix metalloproteinase-3 (MMP-3). Our data suggest that strategies aimed at the inhibition of syndecan-4 will be of great value for the treatment of cartilage damage in osteoarthritis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Glasson, S.S. et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 434, 644–648 (2005).
Stanton, H. et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 434, 648–652 (2005).
Gao, G. et al. ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol–anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J. Biol. Chem. 279, 10042–10051 (2004).
Brooks, P.M. The burden of musculoskeletal disease–a global perspective. Clin. Rheumatol. 25, 778–781 (2006).
Poole, A.R. et al. Proteolysis of the collagen fibril in osteoarthritis. Biochem. Soc. Symp. 70, 115–123 (2003).
Pattoli, M.A., MacMaster, J.F., Gregor, K.R. & Burke, J.R. Collagen and aggrecan degradation is blocked in interleukin-1–treated cartilage explants by an inhibitor of IκB kinase through suppression of metalloproteinase expression. J. Pharmacol. Exp. Ther. 315, 382–388 (2005).
Tortorella, M.D. et al. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science 284, 1664–1666 (1999).
Abbaszade, I. et al. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J. Biol. Chem. 274, 23443–23450 (1999).
Bondeson, J., Wainwright, S., Hughes, C. & Caterson, B. The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin. Exp. Rheumatol. 26, 139–145 (2008).
Song, R.H. et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 56, 575–585 (2007).
Tortorella, M.D., Liu, R.Q., Burn, T., Newton, R.C. & Arner, E. Characterization of human aggrecanase 2 (ADAM-TS5): substrate specificity studies and comparison with aggrecanase 1 (ADAM-TS4). Matrix Biol. 21, 499–511 (2002).
Tortorella, M.D. et al. ADAMTS-4 (aggrecanase-1): N-terminal activation mechanisms. Arch. Biochem. Biophys. 444, 34–44 (2005).
Wang, P. et al. Proprotein convertase furin interacts with and cleaves pro-ADAMTS4 (Aggrecanase-1) in the trans-Golgi network. J. Biol. Chem. 279, 15434–15440 (2004).
Longpré, J.M. et al. Characterization of proADAMTS5 processing by proprotein convertases. Int. J. Biochem. Cell Biol. 41, 1116–1126 (2009).
Tkachenko, E., Rhodes, J.M. & Simons, M. Syndecans: new kids on the signaling block. Circ. Res. 96, 488–500 (2005).
Molténi, A., Modrowski, D., Hott, M. & Marie, P.J. Differential expression of fibroblast growth factor receptor-1, -2, and -3 and syndecan-1, -2, and -4 in neonatal rat mandibular condyle and calvaria during osteogenic differentiation in vitro. Bone 24, 337–347 (1999).
Cornelison, D.D., Filla, M.S., Stanley, H.M., Rapraeger, A.C. & Olwin, B.B. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev. Biol. 239, 79–94 (2001).
Echtermeyer, F. et al. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J. Clin. Invest. 107, R9–R14 (2001).
Lim, S.T., Longley, R.L., Couchman, J.R. & Woods, A. Direct binding of syndecan-4 cytoplasmic domain to the catalytic domain of protein kinase C α (PKC α) increases focal adhesion localization of PKC α. J. Biol. Chem. 278, 13795–13802 (2003).
Wilcox-Adelman, S.A., Denhez, F. & Goetinck, P.F. Syndecan-4 modulates focal adhesion kinase phosphorylation. J. Biol. Chem. 277, 32970–32977 (2002).
Saoncella, S. et al. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc. Natl. Acad. Sci. USA 96, 2805–2810 (1999).
Barre, P.E., Redini, F., Boumediene, K., Vielpeau, C. & Pujol, J.P. Semiquantitative reverse transcription-polymerase chain reaction analysis of syndecan-1 and -4 messages in cartilage and cultured chondrocytes from osteoarthritic joints. Osteoarthritis Cartilage 8, 34–43 (2000).
Clements, K.M. et al. Gene deletion of either interleukin-1β, interleukin-1β-converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial meniscectomy. Arthritis Rheum. 48, 3452–3463 (2003).
Glasson, S.S. et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4–knockout mice. Arthritis Rheum. 50, 2547–2558 (2004).
Kamekura, S. et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage 13, 632–641 (2005).
Blom, A.B. et al. Crucial role of macrophages in matrix metalloproteinase–mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum. 56, 147–157 (2007).
Mankin, H.J., Dorfman, H., Lippiello, L. & Zarins, A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Joint Surg. Am. 53, 523–537 (1971).
Ostergaard, K., Andersen, C.B., Petersen, J., Bendtzen, K. & Salter, D.M. Validity of histopathological grading of articular cartilage from osteoarthritic knee joints. Ann. Rheum. Dis. 58, 208–213 (1999).
Pap, G. et al. Development of osteoarthritis in the knee joints of Wistar rats after strenuous running exercise in a running wheel by intracranial self-stimulation. Pathol. Res. Pract. 194, 41–47 (1998).
Acknowledgements
We would like to thank A. Forsberg, S. Niehues, S. Ecklebe and K. Reher for technical assistance. This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft; Pa689/7-1 to T.P. and F.E. and Th667/6-1 to G.T. and C.H.), the Collaborative Research Centres (SFB 492; project B18 to R.D. and B19 to T.P.) and Deutsche Arthrosehilfe e.V (p77-a117-Rüther-EP2-fuer1-knie-ko—49k-2006-7 to M.F.).
Author information
Authors and Affiliations
Contributions
F.E. designed and performed all Sdc4−/− experiments and wrote the manuscript; J.B. performed all anti-Sdc4-Ab and Mmp3 inhibition experiments; R.D. performed collagen X stainings and northern blot experiments; I.M. performed the surgical induced osteoarthritis in the mice; K.N. performed syndecan-4–specific antibody injections and the MAPK assays; M.F. provided the human osteoarthritis samples; Y.J.L. and Y.W.S. provided the rat osteoarthritis model; C.H. performed the real-time gene expression experiments; G.T. participated in evaluating the data; and T.P. participated in data analysis, directed the project and wrote the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 (PDF 140 kb)
Rights and permissions
About this article
Cite this article
Echtermeyer, F., Bertrand, J., Dreier, R. et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med 15, 1072–1076 (2009). https://doi.org/10.1038/nm.1998
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1998
This article is cited by
-
The role of WWP1 and WWP2 in bone/cartilage development and diseases
Molecular and Cellular Biochemistry (2024)
-
Xylosyltransferase I mediates the synthesis of proteoglycans with long glycosaminoglycan chains and controls chondrocyte hypertrophy and collagen fibers organization of in the growth plate
Cell Death & Disease (2023)
-
Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis
Advances in Rheumatology (2022)
-
Cholesterol-induced LRP3 downregulation promotes cartilage degeneration in osteoarthritis by targeting Syndecan-4
Nature Communications (2022)
-
The miR-302c/transforming growth factor-β receptor type-2 axis modulates interleukin-1β-induced degenerative changes in osteoarthritic chondrocytes
Journal of Cell Communication and Signaling (2022)