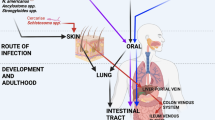
Abstract
Helminths induce potent T helper 2 (TH2)-type immune responses that can mediate worm expulsion, but the role of this response in controlling the acute tissue damage caused by migrating multicellular parasites through vital tissues remains uncertain. We used a helminth infection model in which parasitic nematode larvae migrate transiently through the lung, resulting in hemorrhage and inflammation. We found that IL-17 initially contributed to inflammation and lung damage, whereas subsequent IL-4 receptor (IL-4R) signaling reduced elevations in IL-17 mRNA levels, enhanced the expression of insulin-like growth factor 1 (IGF-1) and IL-10 and stimulated the development of M2 macrophages, all of which contributed to the rapid resolution of tissue damage. These studies indicate an essential role for TH2-type immune responses in mediating acute wound healing during helminth infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Anthony, R.M. et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 12, 955–960 (2006).
Liu, Q. et al. B cells have distinct roles in host protection against different nematode parasites. J. Immunol. 184, 5213–5223 (2010).
Herbert, D.R. et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J. Exp. Med. 206, 2947–2957 (2009).
Zhao, A. et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J. Immunol. 171, 948–954 (2003).
Enobe, C.S. et al. Early stages of Ascaris suum induce airway inflammation and hyperreactivity in a mouse model. Parasite Immunol. 28, 453–461 (2006).
Sakamoto, T. & Gutierrez, C. Pulmonary complications of cystic echinococcosis in children in Uruguay. Pathol. Int. 55, 497–503 (2005).
Girod, N., Brown, A., Pritchard, D.I. & Billett, E.E. Successful vaccination of BALB/c mice against human hookworm (Necator americanus): the immunological phenotype of the protective response. Int. J. Parasitol. 33, 71–80 (2003).
Anthony, R.M., Rutitzky, L.I., Urban, J.F. Jr., Stadecker, M.J. & Gause, W.C. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 7, 975–987 (2007).
McNeil, K.S., Knox, D.P. & Proudfoot, L. Anti-inflammatory responses and oxidative stress in Nippostrongylus brasiliensis-induced pulmonary inflammation. Parasite Immunol. 24, 15–22 (2002).
Loke, P. et al. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J. Immunol. 179, 3926–3936 (2007).
Martin, P. & Leibovich, S.J. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 15, 599–607 (2005).
Shirey, K.A. et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R alpha-, TLR4-, and IFN-beta-dependent. Mucosal Immunol. 3, 291–300 (2010).
Nembrini, C., Marsland, B.J. & Kopf, M. IL-17-producing T cells in lung immunity and inflammation. J. Allergy Clin. Immunol. 123, 986–994 (2009).
Laan, M. et al. Neutrophil recruitment by human IL-17 via C–X-C chemokine release in the airways. J. Immunol. 162, 2347–2352 (1999).
Finkelman, F.D., Hogan, S.P., Hershey, G.K., Rothenberg, M.E. & Wills-Karp, M. Importance of cytokines in murine allergic airway disease and human asthma. J. Immunol. 184, 1663–1674 (2010).
Cooper, A.M. IL-17 and anti-bacterial immunity: protection versus tissue damage. Eur. J. Immunol. 39, 649–652 (2009).
Edgerton, C. et al. IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin. Immunol. 130, 313–321 (2009).
Steinman, L. A rush to judgment on Th17. J. Exp. Med. 205, 1517–1522 (2008).
Seno, H. et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc. Natl. Acad. Sci. USA 106, 256–261 (2009).
Peranteau, W.H. et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J. Invest. Dermatol. 128, 1852–1860 (2008).
Bliss, S.K., Alcaraz, A. & Appleton, J.A. IL-10 prevents liver necrosis during murine infection with Trichinella spiralis. J. Immunol. 171, 3142–3147 (2003).
Apewokin, S., Steciuk, M., Griffin, S. & Jhala, D. Strongyloides hyperinfection diagnosed by bronchoalveolar lavage in an immunocompromized host. Cytopathology 21, 345–347 (2010).
Kolosionek, E., Crosby, A., Harhay, M.O., Morrell, N. & Butrous, G. Pulmonary vascular disease associated with schistosomiasis. Expert Rev. Anti Infect. Ther. 8, 1467–1473 (2010).
Patel, N., Kreider, T., Urban, J.F. Jr. & Gause, W.C. Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites. Int. J. Parasitol. 39, 13–21 (2009).
Herbert, D.R. et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20, 623–635 (2004).
Brunet, L.R., Finkelman, F.D., Cheever, A.W., Kopf, M.A. & Pearce, E.J. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J. Immunol. 159, 777–785 (1997).
Brunet, L.R., Dunne, D.W. & Pearce, E.J. Cytokine Interaction and Immune Responses during Schistosoma mansoni Infection. Parasitol. Today 14, 422–427 (1998).
Gause, W.C., Urban, J.F. Jr. & Stadecker, M.J. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 24, 269–277 (2003).
Mohrs, M., Shinkai, K., Mohrs, K. & Locksley, R.M. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15, 303–311 (2001).
Voehringer, D., Reese, T.A., Huang, X., Shinkai, K. & Locksley, R.M. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J. Exp. Med. 203, 1435–1446 (2006).
Reece, J.J., Siracusa, M.C. & Scott, A.L. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect. Immun. 74, 4970–4981 (2006).
Marsland, B.J., Kurrer, M., Reissmann, R., Harris, N.L. & Kopf, M. Nippostrongylus brasiliensis infection leads to the development of emphysema associated with the induction of alternatively activated macrophages. Eur. J. Immunol. 38, 479–488 (2008).
Li, B. et al. Pulmonary epithelial CCR3 promotes LPS-induced lung inflammation by mediating release of IL-8. J. Cell. Physiol. 226, 2398–2405 (2011).
Krzyzaniak, M. et al. Burn-induced acute lung injury requires a functional toll-like receptor 4. Shock 36, 24–29 (2011).
Massberg, S. et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 16, 887–896 (2010).
Rickel, E.A. et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J. Immunol. 181, 4299–4310 (2008).
Rom, W.N. et al. Alveolar macrophages release an insulin-like growth factor I-type molecule. J. Clin. Invest. 82, 1685–1693 (1988).
Wynes, M.W., Frankel, S.K. & Riches, D.W. IL-4-induced macrophage-derived IGF-I protects myofibroblasts from apoptosis following growth factor withdrawal. J. Leukoc. Biol. 76, 1019–1027 (2004).
Toulon, A. et al. A role for human skin-resident T cells in wound healing. J. Exp. Med. 206, 743–750 (2009).
Kreider, T., Anthony, R.M., Urban, J.F. Jr. & Gause, W.C. Alternatively activated macrophages in helminth infections. Curr. Opin. Immunol. 19, 448–453 (2007).
Mirza, R., DiPietro, L.A. & Koh, T.J. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am. J. Pathol. 175, 2454–2462 (2009).
Cailhier, J.F. et al. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J. Immunol. 174, 2336–2342 (2005).
Herbert, D.R. et al. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J. Immunol. 184, 6438–6446 (2010).
Sandler, N.G., Mentink-Kane, M.M., Cheever, A.W. & Wynn, T.A. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J. Immunol. 171, 3655–3667 (2003).
Balic, A., Harcus, Y.M., Taylor, M.D., Brombacher, F. & Maizels, R.M. IL-4R signaling is required to induce IL-10 for the establishment of T(h)2 dominance. Int. Immunol. 18, 1421–1431 (2006).
Gillery, P., Leperre, A., Maquart, F.X. & Borel, J.P. Insulin-like growth factor-I (IGF-I) stimulates protein synthesis and collagen gene expression in monolayer and lattice cultures of fibroblasts. J. Cell. Physiol. 152, 389–396 (1992).
Hesse, M. et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 167, 6533–6544 (2001).
Martinez, F.O., Helming, L. & Gordon, S. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483 (2009).
Pesce, J.T. et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 5, e1000371 (2009).
Mosser, D.M. & Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Varin, A., Mukhopadhyay, S., Herbein, G. & Gordon, S. Alternative activation of macrophages by IL-4 impairs phagocytosis of pathogens but potentiates microbial-induced signalling and cytokine secretion. Blood 115, 353–362 (2010).
Lee, J.J. & Lee, N.A. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin. Exp. Allergy 35, 986–994 (2005).
Asti, C. et al. Lipopolysaccharide-induced lung injury in mice. I. Concomitant evaluation of inflammatory cells and haemorrhagic lung damage. Pulm. Pharmacol. Ther. 13, 61–69 (2000).
Dovi, J.V., He, L.K. & DiPietro, L.A. Accelerated wound closure in neutrophil-depleted mice. J. Leukoc. Biol. 73, 448–455 (2003).
Simpson, D.M. & Ross, R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J. Clin. Invest. 51, 2009–2023 (1972).
Pesce, J.T. et al. Neutrophils clear bacteria associated with parasitic nematodes augmenting the development of an effective Th2-type response. J. Immunol. 180, 464–474 (2008).
Daley, J.M., Thomay, A.A., Connolly, M.D., Reichner, J.S. & Albina, J.E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83, 64–70 (2008).
Pribul, P.K. et al. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J. Virol. 82, 4441–4448 (2008).
Mionnet, C. et al. CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung. Nat. Med. 16, 1305–1312 (2010).
Kim, J.H. et al. Inhibition of matrix metalloproteinase-9 prevents neutrophilic inflammation in ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L580–L587 (2006).
Acknowledgements
This work was supported by US National Institutes of Health grants AI031678.
Author information
Authors and Affiliations
Contributions
F.C. performed the majority of the experiments and contributed to experimental design. Z.L. contributed to the initial experimental design and essential experiments before leaving the laboratory. W.W. performed experiments with F.C., particularly the flow cytometry experiments, and contributed to the experimental design. C.R. performed experiments with Z.L. and F.C. and contributed to the experimental design. S.B. performed experiments with F.C. and helped develop techniques for tracking parasites. A.M. performed experiments with F.C., maintained and genotyped mouse strains and also prepared parasites for inoculation. N.V.R. provided fresh clodronate liposomes for in vivo experiments and provided advice for use of this reagent for macrophage depletion. J.F.U. maintained parasite stocks and provided them as needed and gave crucial input to the experimental design. T.A.W. provided immunodeficient mice for experiments and gave important input to the experimental design and writing of the paper. W.C.G. designed most of research project, provided oversight of experiments and wrote the majority of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 870 kb)
Rights and permissions
About this article
Cite this article
Chen, F., Liu, Z., Wu, W. et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med 18, 260–266 (2012). https://doi.org/10.1038/nm.2628
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2628
This article is cited by
-
Evaluation of the immunoprotective effects of eight recombinant proteins from Baylisascaris schroederi in mice model
Parasites & Vectors (2023)
-
IL-4/IL-4 Ab complex enhances the accumulation of both antigen-specific and bystander CD8 T cells in mouse lungs infected with influenza A virus
Laboratory Animal Research (2023)
-
The impact of helminth-induced immunity on infection with bacteria or viruses
Veterinary Research (2023)
-
Single cell transcriptomics clarifies the basophil differentiation trajectory and identifies pre-basophils upstream of mature basophils
Nature Communications (2023)
-
From mucosal infection to successful cancer immunotherapy
Nature Reviews Urology (2023)