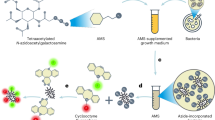
Abstract
The intestine is densely populated by anaerobic commensal bacteria. These microorganisms shape immune system development, but understanding of host–commensal interactions is hampered by a lack of tools for studying the anaerobic intestinal environment. We applied metabolic oligosaccharide engineering and bioorthogonal click chemistry to label various commensal anaerobes, including Bacteroides fragilis, a common and immunologically important commensal. We studied the dissemination of B. fragilis after acute peritonitis and characterized the interactions of the intact microbe and its polysaccharide components in myeloid and B cell lineages. We were able to assess the distribution and colonization of labeled B. fragilis along the intestine, as well as niche competition after coadministration of multiple species of the microbiota. We also fluorescently labeled nine additional commensals (eight anaerobic and one microaerophilic) from three phyla common in the gut—Bacteroidetes, Firmicutes and Proteobacteria—as well as one aerobic pathogen (Staphylococcus aureus). This strategy permits visualization of the anaerobic microbial niche by various methods, including intravital two-photon microscopy and non-invasive whole-body imaging, and can be used to study microbial colonization and host–microbe interactions in real time.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Turnbaugh, P.J. et al. The human microbiome project. Nature 449, 804–810 (2007).
Hooper, L.V., Littman, D.R. & Macpherson, A.J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Ivanov, I.I. & Littman, D.R. Modulation of immune homeostasis by commensal bacteria. Curr. Opin. Microbiol. 14, 106–114 (2011).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013).
Ahern, P.P., Faith, J.J. & Gordon, J.I. Mining the human gut microbiota for effector strains that shape the immune system. Immunity 40, 815–823 (2014).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Ivanov, I.I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498, 33 (2009).
An, D. et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156, 123–133 (2014).
Dasgupta, S. & Kasper, D.L. Relevance of commensal microbiota in the treatment and prevention of inflammatory bowel disease. Inflamm. Bowel Dis. 19, 2478–2489 (2013).
Yurkovetskiy, L.A., Pickard, J.M. & Chervonsky, A.V. Microbiota and autoimmunity: exploring new avenues. Cell Host Microbe 17, 548–552 (2015).
Sommer, F. & Backhed, F. The gut microbiota—masters of host development and physiology. Nat. Rev. Microbiol. 11, 227–238 (2013).
Surana, N.K. & Kasper, D.L. The yin yang of bacterial polysaccharides: lessons learned from B. fragilis PSA. Immunol. Rev. 245, 13–26 (2012).
Mazmanian, S.K., Liu, C.H., Tzianabos, A.O. & Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118 (2005).
Krinos, C.M. et al. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature 414, 555–558 (2001).
Coyne, M.J., Chatzidaki-Livanis, M., Paoletti, L.C. & Comstock, L.E. Role of glycan synthesis in colonization of the mammalian gut by the bacterial symbiont Bacteroides fragilis. Proc. Natl. Acad. Sci. USA 105, 13099–13104 (2008).
Dasgupta, S., Erturk-Hasdemir, D., Ochoa-Reparaz, J., Reinecker, H.C. & Kasper, D.L. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe 15, 413–423 (2014).
Ochoa-Repáraz, J. et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 3, 487–495 (2010).
Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544 (1998).
Craggs, T.D. Green fluorescent protein: structure, folding and chromophore maturation. Chem. Soc. Rev. 38, 2865–2875 (2009).
Boyce, M. & Bertozzi, C.R. Bringing chemistry to life. Nat. Methods 8, 638–642 (2011).
Sletten, E.M. & Bertozzi, C.R. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem. Int. Edn. Engl. 48, 6974–6998 (2009).
Dehnert, K.W. et al. Metabolic labeling of fucosylated glycans in developing zebrafish. ACS Chem. Biol. 6, 547–552 (2011).
Dumont, A., Malleron, A., Awwad, M., Dukan, S. & Vauzeilles, B. Click-mediated labeling of bacterial membranes through metabolic modification of the lipopolysaccharide inner core. Angew. Chem. Int. Edn. Engl. 51, 3143–3146 (2012).
Kaewsapsak, P., Esonu, O. & Dube, D.H. Recruiting the host's immune system to target Helicobacter pylori's surface glycans. ChemBioChem 14, 721–726 (2013).
Cobb, B.A. & Kasper, D.L. Characteristics of carbohydrate antigen binding to the presentation protein HLA-DR. Glycobiology 18, 707–718 (2008).
Hawkins, E.D. et al. Measuring lymphocyte proliferation, survival and differentiation using CFSE time-series data. Nat. Protoc. 2, 2057–2067 (2007).
Troy, E.B., Carey, V.J., Kasper, D.L. & Comstock, L.E. Orientations of the Bacteroides fragilis capsular polysaccharide biosynthesis locus promoters during symbiosis and infection. J. Bacteriol. 192, 5832–5836 (2010).
Coyne, M.J. et al. Polysaccharide biosynthesis locus required for virulence of Bacteroides fragilis. Infect. Immun. 69, 4342–4350 (2001).
Coyne, M.J., Weinacht, K.G., Krinos, C.M. & Comstock, L.E. Mpi recombinase globally modulates the surface architecture of a human commensal bacterium. Proc. Natl. Acad. Sci. USA 100, 10446–10451 (2003).
Nord, C.E. Incidence and significance of intraperitoneal aerobic and anaerobic bacteria. Clin. Ther. 12, 9–20 (1990).
Millet, Y.A. et al. Insights into Vibrio cholerae intestinal colonization from monitoring fluorescently labeled bacteria. PLoS Pathog. 10, e1004405 (2014).
Farache, J., Zigmond, E., Shakhar, G. & Jung, S. Contributions of dendritic cells and macrophages to intestinal homeostasis and immune defense. Immunol. Cell Biol. 91, 232–239 (2013).
Dethlefsen, L., McFall-Ngai, M. & Relman, D.A. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature 449, 811–818 (2007).
Martinez-Pomares, L. & Gordon, S. CD169+ macrophages at the crossroads of antigen presentation. Trends Immunol. 33, 66–70 (2012).
Cyster, J.G. B cell follicles and antigen encounters of the third kind. Nat. Immunol. 11, 989–996 (2010).
Vinuesa, C.G., Linterman, M.A., Goodnow, C.C. & Randall, K.L. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol. Rev. 237, 72–89 (2010).
Acknowledgements
We thank the Neurobiology Imaging Facility for consultation and instrument availability that supported this work. This facility is supported in part by the Neural Imaging Center as part of NINDS P30 Core Center grant NS072030. We also thank N. Barteneva of the Flow and Imaging Cytometry Resource for help in whole-body imaging on the IVIS Spectrum (funded by grants NIH 1 S10 OD016401 and BCH/PCMM); J. Czupryna and M. Guerra from PerkinElmer for consultation and expert advice; C. Araneo (Division of Immunology Flow Cytometry Core Facility, HMS) and L. Comstock for helpful discussions, comments and reagents; and members of the Kasper and von Andrian labs for helpful discussions. This work was also partly supported by the US National Institutes of Health (grants PO1 AI1112521, RO1 AI111595 (to U.H.v.A.) and 5T32 HL066987 (to D.A.)). Additional support to U.H.v.A. was provided by U19AI095261 and the Ragon Institute at MGH, MIT and Harvard. N.G.-Z. was supported by an EMBO Long-Term Fellowship (ALTF 251-2011), the Human Frontiers Science Program (LT000079/2012), a UNESCO L'Oreal National and International Women in Science Award, a Fulbright Award and the Weizmann Institute of Science–National Postdoctoral Award Program for Advancing Women in Science. J.E.H. was supported by the Cancer Research Institute Irvington Fellowship Program. N.C.R. was supported by a departmental Kirschstein-NRSA training grant.
Author information
Authors and Affiliations
Contributions
N.G.-Z. and D.A. designed the experiments, analyzed the data and wrote the manuscript with help from J.E.H. and N.C.R. D.E.-H. and S.D. provided experimental help and expertise. D.L.K. supervised the study, edited the manuscript and provided helpful comments, with assistance from U.H.v.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 & Supplementary Table 1 (PDF 7615 kb)
41591_2015_BFnm3929_MOESM36_ESM.mp4
Non-invasive 3D whole-body longitudinal optical fluorescence imaging of live mice after oral administration of labeled B. fragilis (MP4 1612 kb)
Rights and permissions
About this article
Cite this article
Geva-Zatorsky, N., Alvarez, D., Hudak, J. et al. In vivo imaging and tracking of host–microbiota interactions via metabolic labeling of gut anaerobic bacteria. Nat Med 21, 1091–1100 (2015). https://doi.org/10.1038/nm.3929
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3929
This article is cited by
-
Omics for deciphering oral microecology
International Journal of Oral Science (2024)
-
Intravital imaging of immune responses in intestinal inflammation
Inflammation and Regeneration (2023)
-
Combinatorial fluorescent labeling of live anaerobic bacteria via the incorporation of azide-modified sugars into newly synthesized macromolecules
Nature Protocols (2023)
-
Using click chemistry to study microbial ecology and evolution
ISME Communications (2023)
-
Technical pipeline for screening microbial communities as a function of substrate specificity through fluorescent labelling
Communications Biology (2022)