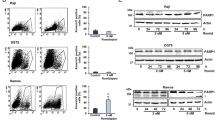
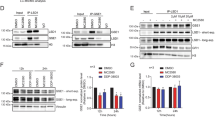
Abstract
Chromatin is a dynamic macromolecular structure epigenetically modified to regulate specific gene expression. Altered chromatin function can lead to aberrant expression of growth regulators and may, ultimately, cause cancer. That many human diseases have epigenetic etiology has stimulated the development of 'epigenetic' therapies. Inhibitors of histone deacetylases (HDACIs) induce proliferation arrest, maturation and apoptosis of cancer cells, but not normal cells, in vitro and in vivo, and are currently being tested in clinical trials1,2,3,4,5. We investigated the mechanism(s) underlying this tumor selectivity. We report that HDACIs induce, in addition to p21, expression of TRAIL (Apo2L, TNFSF10) by directly activating the TNFSF10 promoter, thereby triggering tumor-selective death signaling in acute myeloid leukemia (AML) cells and the blasts of individuals with AML. RNA interference revealed that the induction of p21, TRAIL and differentiation are separable activities of HDACIs. HDACIs induced proliferation arrest, TRAIL-mediated apoptosis and suppression of AML blast clonogenicity irrespective of French-American-British (FAB) classification status, karyotype and immunophenotype. No apoptosis was seen in normal CD34+ progenitor cells. Our results identify TRAIL as a mediator of the anticancer action of HDACIs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Marks, P. et al. Histone deacetylases and cancer: causes and therapies. Nat. Rev. Cancer 1, 194–202 (2001).
Johnstone, R.W. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 1, 287–299 (2002).
Egger, G., Liang, G., Aparicio, A. & Jones, P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature 429, 457–463 (2004).
Vigushin, D.M. & Coombes, R.C. Targeted histone deacetylase inhibition for cancer therapy. Curr. Cancer Drug Targets 4, 205–218 (2004).
Minucci, S., Nervi, C., Lo Coco, F. & Pelicci, P.G. Histone deacetylases: a common molecular target for differentiation treatment of acute myeloid leukemias? Oncogene 20, 3110–3115 (2001).
Ferrara, F.F. et al. Histone deacetylase-targeted treatment restores retinoic acid signaling and differentiation in acute myeloid leukemia. Cancer Res. 61, 2–7 (2001).
Gottlicher, M. et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 20, 6969–6978 (2001).
Phiel, C.J. et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 276, 36734–36741 (2001).
Amin, H.M., Saeed, S. & Alkan, S. Histone deacetylase inhibitors induce caspase-dependent apoptosis and downregulation of daxx in acute promyelocytic leukaemia with t(15;17). Br. J. Haematol. 115, 287–297 (2001).
Suzuki, T. et al. Synthesis and histone deacetylase inhibitory activity of new benzamide derivatives. J. Med. Chem. 42, 3001–3003 (1999).
Saito, A. et al. A synthetic inhibitor of histone deacetylase, MS-27–275, with marked in vivo antitumor activity against human tumors. Proc. Natl. Acad. Sci. USA 96, 4592–4597 (1999).
Rosato, R.R., Almenara, J.A. & Grant, S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res. 63, 3637–3645 (2003).
Richon, V.M., Sandhoff, T.W., Rifkind, R.A. & Marks, P.A. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl. Acad. Sci. USA 97, 10014–10019 (2000).
Glaser, K.B. et al. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol. Cancer Ther. 2, 151–163 (2003).
Sasakawa, Y. et al. Effects of FK228, a novel histone deacetylase inhibitor, on human lymphoma U-937 cells in vitro and in vivo. Biochem. Pharmacol. 64, 1079–1090 (2002).
Clarke, N., Jimenez-Lara, A.M., Voltz, E. & Gronemeyer, H. Tumor suppressor IRF-1 mediates retinoid and interferon anticancer signaling to death ligand TRAIL. EMBO J. 23, 3051–3060 (2004).
Ammanamanchi, S., Freeman, J.W. & Brattain, M.G. Acetylated sp3 is a transcriptional activator. J. Biol. Chem. 278, 35775–35780 (2003).
Bouwman, P. & Philipsen, S. Regulation of the activity of Sp1-related transcription factors. Mol. Cell. Endocrinol. 195, 27–38 (2002).
Braun, H., Koop, R., Ertmer, A., Nacht, S. & Suske, G. Transcription factor Sp3 is regulated by acetylation. Nucleic Acids Res. 29, 4994–5000 (2001).
Walczak, H. et al. Tumoricidal activity of tumor necrosis factor-related apoptosis- inducing ligand in vivo. Nat. Med. 5, 157–163 (1999).
Litwack, G. (ed.) Vitamins and Hormones: TRAIL, 448 pages (Academic Press, 2004).
Kelley, S.K. & Ashkenazi, A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr. Opin. Pharmacol. 4, 333–339 (2004).
Altucci, L. & Gronemeyer, H. The promise of retinoids to fight against cancer. Nat. Rev. Cancer 1, 181–193 (2001).
Altucci, L. et al. Retinoic acid-induced apoptosis in leukemia cells is mediated by paracrine action of tumor-selective death ligand TRAIL. Nat. Med. 7, 680–686 (2001).
Ryu, H. et al. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc. Natl. Acad. Sci. USA 100, 4281–4286 (2003).
Camarero, N., Nadal, A., Barrero, M.J., Haro, D. & Marrero, P.F. Histone deacetylase inhibitors stimulate mitochondrial HMG-CoA synthase gene expression via a promoter proximal Sp1 site. Nucleic Acids Res. 31, 1693–1703 (2003).
Won, J., Yim, J. & Kim, T.K. Sp1 and Sp3 recruit histone deacetylase to repress transcription of human telomerase reverse transcriptase (hTERT) promoter in normal human somatic cells. J. Biol. Chem. 277, 38230–38238 (2002).
Rosato, R.R., Almenara, J.A., Dai, Y. & Grant, S. Simultaneous activation of the intrinsic and extrinsic pathways by histone deacetylase (HDAC) inhibitors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically induces mitochondrial damage and apoptosis in human leukemia cells. Mol. Cancer Ther. 2, 1273–1284 (2003).
Brummelkamp, T.R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002).
Shiohara, M. et al. Effects of novel RAR- and RXR-selective retinoids on myeloid leukemic proliferation and differentiation in vitro. Blood 93, 2057–2066 (1999).
Acknowledgements
This work is dedicated to the memory of T. Battista. We are grateful to C. Erb and J.M. Garnier for modifying the pSUPER vector, a gift of R. Bernards, for permanent RNAi, and construction and validation of the siRNA vectors, E. Wilhelm for ChIP assays, A. Scognamiglio, C. Scafoglio and M. DeSimone for discussions, A. Cuomo and R. Verde for technical assistance. A.N. and N.C. were supported by the European Union, E.V. by a fellowship from the Ministère de la Recherche et Technologie and E.G. by a fellowship from the Ligue Nationale Contre le Cancer. This work was supported by grants from the European Union (QLG1-CT2000-01935 and QLK3-CT2002-02029), Regione Campania, Ministero della Salute R.F. 02/184, Associazione Italiana per la Ricerca sul Cancro, Ministero dell'Istruzione, Università e Ricerca (PRIN 2001067229_002, PRIN 2002067514_002, PRIN 2004D55579 and FIRB RBNE0157EH), the Italian-French GALILEO-VINCI program, Fondation de France, Association for International Cancer Research, Association pour la Recherche sur le Cancer, Université Louis Pasteur, Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, and Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Three HDAC inhibitors induce TRAIL mRNA and protein expression in different myeloid cell lines. (PDF 32 kb)
Supplementary Fig. 2
Permanent RNA interference to reveal the specific roles of TRAIL and p21, kinetics of differentiation and apoptosis markers, and TRAIL promoter analysis by ChIP assays. (PDF 82 kb)
Supplementary Fig. 3
MS275-induced TRAIL expression precedes differentiation and is responsible for antitumor activity of MS275 in vivo. (PDF 52 kb)
Supplementary Fig. 4
Effect of MS275 exposure on TRAIL mRNA and protein expression in AML patients' blasts and normal CD34+ progenitors. (PDF 27 kb)
Rights and permissions
About this article
Cite this article
Nebbioso, A., Clarke, N., Voltz, E. et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat Med 11, 77–84 (2005). https://doi.org/10.1038/nm1161
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1161
This article is cited by
-
Bayesian variable selection for high-dimensional data with an ordinal response: identifying genes associated with prognostic risk group in acute myeloid leukemia
BMC Bioinformatics (2021)
-
Harnessing the HDAC–histone deacetylase enzymes, inhibitors and how these can be utilised in tissue engineering
International Journal of Oral Science (2019)
-
Differentiation therapy revisited
Nature Reviews Cancer (2018)
-
Developing TRAIL/TRAIL death receptor-based cancer therapies
Cancer and Metastasis Reviews (2018)
-
Time-resolved analysis of DNA-protein interactions in living cells by UV laser pulses
Scientific Reports (2017)