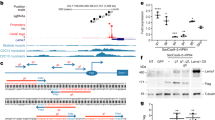
Abstract
The muscular dystrophies are a heterogeneous group of disorders for which there are currently no cures. Oculopharyngeal muscular dystrophy (OPMD) is an autosomal dominant late-onset, progressive disease that generally presents in the fifth or sixth decade with dysphagia, ptosis and proximal limb weakness. OPMD is caused by the abnormal expansion of a (GCG)n trinucleotide repeat in the coding region of the poly-(A) binding protein nuclear 1 (PABPN1) gene1. In unaffected individuals, (GCG)6 codes for the first six alanines in a homopolymeric stretch of ten alanines. In most individuals with OPMD this (GCG)6 repeat is expanded to (GCG)8–13, leading to a stretch of 12–17 alanines in mutant PABPN1. PABPN1 with an expanded polyalanine tract forms aggregates consisting of tubular filaments within the nuclei of skeletal muscle fibers2,3,4. We have developed a transgenic mouse model of OPMD that manifests progressive muscle weakness accompanied by intranuclear aggregates and TUNEL-stained nuclei in skeletal muscle fibers. The onset and severity of these abnormalities were substantially delayed and attenuated by doxycycline treatment, which may exert its therapeutic effect by reducing aggregates and by distinct antiapoptotic properties. Doxycycline may represent a safe and feasible therapeutic for this disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brais, B. et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat. Genet. 8, 164–167 (1998).
Tome, F. M, Chateau, D., Helbling-Leclerc, A. & Fardeau, M. Morphological changes in muscle fibers in oculopharyngeal muscular dystrophy. Neuromuscul. Disord. 7, S63–S69 (1997).
Uyama, E. et al. Nuclear accumulation of expanded PABP2 gene product in oculopharyngeal muscular dystrophy. Muscle Nerve 23, 1549–1554 (2000).
Calado, A. et al. Nuclear inclusions in oculopharyngeal muscular dystrophy consist of poly(A) binding protein 2 aggregates which sequester poly(A) RNA. Hum. Mol. Genet. 9, 2321–2328 (2000).
Albrecht, A.N. et al. A molecular pathogenesis for transcription factor associated poly-alanine tract expansions. Hum. Mol. Genet. 13, 2351–2359 (2004).
Abu-Baker, A., Messaed, C., Laganiere, J., Gaspar, C., Brais, B. & Rouleau, G.A. Involvement of the ubiquitin-proteasome pathway and molecular chaperones in oculopharyngeal muscular dystrophy. Hum. Mol. Genet. 12, 2609–2623 (2003).
Bao, Y.P., Cook, L.J., O'Donovan, D., Uyama, E. & Rubinsztein, D.C. Mammalian, yeast, bacterial, and chemical chaperones reduce aggregate formation and death in a cell model of oculopharyngeal muscular dystrophy. J. Biol. Chem. 277, 12263–12269 (2002).
Bao, Y.P., Sarkar, S., Uyama, E. & Rubinsztein, D.C. Mammalian, yeast, bacterial, and chemical chaperones reduce aggregate formation and death in a cell model of oculopharyngeal muscular dystrophy. J. Med. Genet. 41, 47–51 (2004).
Forloni, G., Colombo, L., Girola, L., Tagliavini, F. & Salmona, M. Anti-amyloidogenic activity of tetracyclines: studies in vitro. FEBS Lett. 487, 404–407 (2001).
Chen, M. et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 6, 797–801 (2000).
Wang, X. et al. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc. Natl. Acad. Sci. USA 100, 10483–10487 (2003).
Hino, H. et al. Myopathy phenotype in transgenic mice expressing mutated PABPN1 as a model of oculopharyngeal muscular dystrophy. Hum. Mol. Gen. 13, 181–190 (2004).
Brennan, K.J. & Hardeman, E.C. Quantitative analysis of the human alpha-skeletal actin gene in transgenic mice. J. Biol. Chem. 268, 719–725 (1993).
Tinsley, J.M., Potter, A.C., Phelps, S.R., Fisher, R., Trickett, J.I & Davies, K.E. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature 384, 349–353 (1996).
Fan, X., Dion, P., Laganiere, J., Brais, B. & Rouleau G.A. Oligomerization of polyalanine expanded PABPN1 facilitates nuclear protein aggregation that is associated with cell death. Hum. Mol. Genet. 10, 2341–2351 (2001).
Perez, D. & White, E. TNF-alpha signals apoptosis through a bid-dependent conformational change in Bax that is inhibited by E1B 19K. Mol. Cell. 6, 53–63 (2000).
Wyttenbach, A., Sauvageot, O., Carmichael J., Diaz-Latoud, C., Arrigo A.P. & Rubinsztein D.C. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum. Mol. Genet. 11, 1137–1151 (2002).
Zhu, S. et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 417, 74–78 (2002).
Girgenrath, M., Dominov, J.A., Kostek, C.A. & Miller, J.B. Inhibition of apoptosis improves outcome in a model of congenital muscular dystrophy. J Clin. Invest. 114, 1635–1639 (2004).
Smith, D.L. et al. Minocycline and doxycycline are not beneficial in a model of Huntington's disease. Ann. Neurol. 54, 186–196 (2003).
Nemeth, A. et al. Isolation of genomic and cDNA clones encoding bovine poly(A) binding protein II. Nucleic Acid Res. 23, 4034–4041 (1995).
Rogers, D.C., Fisher, E.M., Brown, S.D., Peters, J., Hunter, A.J. & Martin, J.E. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm. Genome. 8, 711–713 (1997).
Acknowledgements
The authors would like to thank E.Wahle for the kind gift of PABPN1 antibody and PABPN1 constructs, K.E. Davies for the human skeletal muscle actin construct, M. Bobrow for helpful comments and G. Gatward and L. Gilroy for technical assistance. This work was funded by the Wellcome Trust (Senior Fellowship to D.C. Rubinsztein) and a Medical Research Council Programme grant to D.C.R. (with S. Brown).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
OPMD transgenic construct design, expression, and aggregate characterization. (PDF 131 kb)
Supplementary Fig. 2
Phenotypic assessment of OPMD transgenic mice. (PDF 34 kb)
Supplementary Fig. 3
Effects of doxycycline treatment on mouse and cellular models of OPMD. (PDF 49 kb)
Rights and permissions
About this article
Cite this article
Davies, J., Wang, L., Garcia-Oroz, L. et al. Doxycycline attenuates and delays toxicity of the oculopharyngeal muscular dystrophy mutation in transgenic mice. Nat Med 11, 672–677 (2005). https://doi.org/10.1038/nm1242
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1242
This article is cited by
-
CRISPR-Cas9 mediated generation of a conditional poly(A) binding protein nuclear 1 (Pabpn1) mouse model reveals an essential role for hematopoietic stem cells
Scientific Reports (2022)
-
Assessment of PABPN1 nuclear inclusions on a large cohort of patients and in a human xenograft model of oculopharyngeal muscular dystrophy
Acta Neuropathologica (2022)
-
Dopaminergic Projection from Ventral Tegmental Area to Substantia Nigra Pars Reticulata Mediates Chronic Social Defeat Stress–Induced Hypolocomotion
Molecular Neurobiology (2021)
-
Mitochondrial localization of PABPN1 in oculopharyngeal muscular dystrophy
Laboratory Investigation (2019)