Abstract
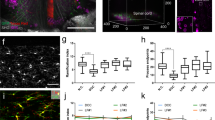
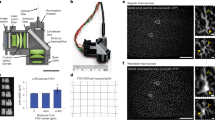
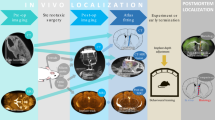
Understanding and treatment of spinal cord pathology is limited in part by a lack of time-lapse in vivo imaging strategies at the cellular level. We developed a chronically implanted spinal chamber and surgical procedure suitable for time-lapse in vivo multiphoton microscopy of mouse spinal cord without the need for repeat surgical procedures. We routinely imaged mice repeatedly for more than 5 weeks postoperatively with up to ten separate imaging sessions and observed neither motor-function deficit nor neuropathology in the spinal cord as a result of chamber implantation. Using this chamber we quantified microglia and afferent axon dynamics after a laser-induced spinal cord lesion and observed massive microglia infiltration within 1 d along with a heterogeneous dieback of axon stumps. By enabling chronic imaging studies over timescales ranging from minutes to months, our method offers an ideal platform for understanding cellular dynamics in response to injury and therapeutic interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kobat, D. et al. Deep tissue multiphoton microscopy using longer wavelength excitation. Opt. Express 17, 13354–13364 (2009).
Christie, R.H. et al. Growth arrest of individual senile plaques in a model of Alzheimer′s disease observed by in vivo multiphoton microscopy. J. Neurosci. 21, 858–864 (2001).
Yan, P. et al. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J. Neurosci. 29, 10706–10714 (2009).
Meyer-Luehmann, M. et al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer′s disease. Nature 451, 720–724 (2008).
Prada, C.M. et al. Antibody-mediated clearance of amyloid-beta peptide from cerebral amyloid angiopathy revealed by quantitative in vivo imaging. J. Neurosci. 27, 1973–1980 (2007).
Kienast, Y. et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 16, 116–122 (2010).
Lam, C.K., Yoo, T., Hiner, B., Liu, Z. & Grutzendler, J. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature 465, 478–482 (2010).
Holtmaat, A. et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4, 1128–1144 (2009).
Yang, G., Pan, F., Parkhurst, C.N., Grutzendler, J. & Gan, W.B. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat. Protoc. 5, 201–208 (2010).
Drew, P.J. et al. Chronic optical access through a polished and reinforced thinned skull. Nat. Methods 7, 981–984 (2010).
Bhatt, D.H., Otto, S.J., Depoister, B. & Fetcho, J.R. Cyclic AMP-induced repair of zebrafish spinal circuits. Science 305, 254–258 (2004).
Kerschensteiner, M., Schwab, M.E., Lichtman, J.W. & Misgeld, T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat. Med. 11, 572–577 (2005).
Davalos, D. et al. Stable in vivo imaging of densely populated glia, axons and blood vessels in the mouse spinal cord using two-photon microscopy. J. Neurosci. Methods 169, 1–7 (2008).
Dibaj, P. et al. NO mediates microglial response to acute spinal cord injury under ATP control in vivo. Glia 58, 1133–1144 (2010).
Johannssen, H.C. & Helmchen, F. In vivo Ca2+ imaging of dorsal horn neuronal populations in mouse spinal cord. J. Physiol. (Lond.) 588, 3397–3402 (2010).
Ylera, B. et al. Chronically CNS-injured adult sensory neurons gain regenerative competence upon a lesion of their peripheral axon. Curr. Biol. 19, 930–936 (2009).
Kim, J.V. et al. Two-photon laser scanning microscopy imaging of intact spinal cord and cerebral cortex reveals requirement for CXCR6 and neuroinflammation in immune cell infiltration of cortical injury sites. J. Immunol. Methods 352, 89–100 (2010).
Dray, C., Rougon, G. & Debarbieux, F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc. Natl. Acad. Sci. USA 106, 9459–9464 (2009).
Farrar, M.J., Wise, F.W., Fetcho, J.R. & Schaffer, C.B. In vivo imaging of myelin in the vertebrate central nervous system using third harmonic generation microscopy. Biophys. J. 100, 1362–1371 (2011).
Crawley, J.N. What's Wrong with My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice (Wiley-Liss, New York, 2000).
Streit, W.J., Walter, S.A. & Pennell, N.A. Reactive microgliosis. Prog. Neurobiol. 57, 563–581 (1999).
Tanaka, T., Ueno, M. & Yamashita, T. Engulfment of axon debris by microglia requires p38 MAPK activity. J. Biol. Chem. 284, 21626–21636 (2009).
Aldskogius, H. & Kozlova, E.N. Central neuron-glial and glial-glial interactions following axon injury. Prog. Neurobiol. 55, 1–26 (1998).
Garcia-Alias, G. et al. Therapeutic time window for the application of chondroitinase ABC after spinal cord injury. Exp. Neurol. 210, 331–338 (2008).
Rolls, A., Shechter, R. & Schwartz, M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 10, 235–241 (2009).
Tator, C.H. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery 59, 957–987 (2006).
Thuret, S., Moon, L.D. & Gage, F.H. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 7, 628–643 (2006).
Seif, G.I., Nomura, H. & Tator, C.H. Retrograde axonal degeneration (“dieback”) in the corticospinal tract after transection injury of the rat spinal cord: a confocal microscopy study. J. Neurotrauma 24, 1513–1528 (2007).
Silver, J., Horn, K.P., Busch, S.A., Hawthorne, A.L. & van Rooijen, N. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J. Neurosci. 28, 9330–9341 (2008).
Xu, H.T., Pan, F., Yang, G. & Gan, W.B. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat. Neurosci. 10, 549–551 (2007).
Acknowledgements
We thank the US National Institutes of Health (DP OD006411 to J.R.F. and R01 EB002019 to C.B.S.) and the National Science and Research Council of Canada (to M.J.F.) for financial support, IMRA America, Inc. for the loan of their FCPA μJewel D-400 laser, J. Siebert for critically reading this manuscript, N. Ellis for his assistance in the machine shop and M. Riccio for his assistance with the MicroCT imaging.
Author information
Authors and Affiliations
Contributions
M.J.F., T.A.C., J.R.F. and C.B.S. conceived and designed the experiments. M.J.F. performed surgeries and imaging experiments, I.M.B. performed behavioral assays, and D.H.S. performed histopathology. M.J.F., I.M.B., J.R.F. and C.B.S. analyzed data. J.R.F., T.A.C. and C.B.S. contributed reagents and materials. M.J.F., J.R.F. and C.B.S. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Notes 1–7, Supplementary Protocol (PDF 1956 kb)
Supplementary Video 1
Rendering of MicroCT image data from a mouse taken 6 d after implantation of the imaging chamber, showing normal spine alignment and no vertebral damage. (MOV 7283 kb)
Supplementary Video 2
Video of a mouse taken 2 weeks after implanting the imaging chamber, showing locomotion, grooming and exploratory behavior. (MOV 7639 kb)
Supplementary Video 3
A series of 2PEF image stacks taken at different times after a laser-induced spinal cord injury, showing GFP-expressing axons (green) and Texas Red-dextran labeled vasculature (red). (MOV 29674 kb)
Rights and permissions
About this article
Cite this article
Farrar, M., Bernstein, I., Schlafer, D. et al. Chronic in vivo imaging in the mouse spinal cord using an implanted chamber. Nat Methods 9, 297–302 (2012). https://doi.org/10.1038/nmeth.1856
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1856
This article is cited by
-
Pathological hemodynamic changes and leukocyte transmigration disrupt the blood–spinal cord barrier after spinal cord injury
Journal of Neuroinflammation (2023)
-
Long-term in vivo imaging of mouse spinal cord through an optically cleared intervertebral window
Nature Communications (2022)
-
Activation of spinal dorsal horn astrocytes by noxious stimuli involves descending noradrenergic signaling
Molecular Brain (2021)
-
In vivo imaging of injured cortical axons reveals a rapid onset form of Wallerian degeneration
BMC Biology (2020)
-
An intravital window to image the colon in real time
Nature Communications (2019)