Abstract
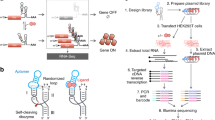
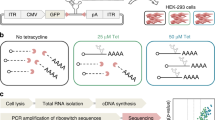
Methods for rapidly assessing sequence-structure-function landscapes and developing conditional gene-regulatory devices are critical to our ability to manipulate and interface with biology. We describe a framework for engineering RNA devices from preexisting aptamers that exhibit ligand-responsive ribozyme tertiary interactions. Our methodology utilizes cell sorting, high-throughput sequencing and statistical data analyses to enable parallel measurements of the activities of hundreds of thousands of sequences from RNA device libraries in the absence and presence of ligands. Our tertiary-interaction RNA devices performed better in terms of gene silencing, activation ratio and ligand sensitivity than optimized RNA devices that rely on secondary-structure changes. We applied our method to build biosensors for diverse ligands and determine consensus sequences that enable ligand-responsive tertiary interactions. These methods advance our ability to develop broadly applicable genetic tools and to elucidate the underlying sequence-structure-function relationships that empower rational design of complex biomolecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Win, M.N. & Smolke, C.D. A modular and extensible RNA-based gene-regulatory platform for engineering cellular function. Proc. Natl. Acad. Sci. USA 104, 14283–14288 (2007).
Wei, K.Y., Chen, Y.Y. & Smolke, C.D. A yeast-based rapid prototype platform for gene control elements in mammalian cells. Biotechnol. Bioeng. 110, 1201–1210 (2013).
Kennedy, A.B., Vowles, J.V., d'Espaux, L. & Smolke, C.D. Protein-responsive ribozyme switches in eukaryotic cells. Nucleic Acids Res. 42, 12306–12321 (2014).
Ausländer, S., Ketzer, P. & Hartig, J.S. A ligand-dependent hammerhead ribozyme switch for controlling mammalian gene expression. Mol. Biosyst. 6, 807–814 (2010).
Wieland, M. & Hartig, J.S. Improved aptazyme design and in vivo screening enable riboswitching in bacteria. Angew. Chem. Int. Edn Engl. 47, 2604–2607 (2008).
Nomura, Y., Zhou, L., Miu, A. & Yokobayashi, Y. Controlling mammalian gene expression by allosteric hepatitis delta virus ribozymes. ACS Synth. Biol. 2, 684–689 (2013).
Wittmann, A. & Suess, B. Selection of tetracycline inducible self-cleaving ribozymes as synthetic devices for gene regulation in yeast. Mol. Biosyst. 7, 2419–2427 (2011).
Klauser, B., Atanasov, J., Siewert, L.K. & Hartig, J.S. Ribozyme-based aminoglycoside switches of gene expression engineered by genetic selection in. S. cerevisiae. ACS Synth. Biol. 15, 516–525 (2015).
Ausländer, S. et al. A general design strategy for protein-responsive riboswitches in mammalian cells. Nat. Methods 11, 1154–1160 (2014).
Win, M.N. & Smolke, C.D. Higher-order cellular information processing with synthetic RNA devices. Science 322, 456–460 (2008).
Galloway, K.E., Franco, E. & Smolke, C.D. Dynamically reshaping signaling networks to program cell fate via genetic controllers. Science 341, 1235005 (2013).
Chen, Y.Y., Jensen, M.C. & Smolke, C.D. Genetic control of mammalian T-cell proliferation with synthetic RNA regulatory systems. Proc. Natl. Acad. Sci. USA 107, 8531–8536 (2010).
Liang, J.C., Chang, A.L., Kennedy, A.B. & Smolke, C.D. A high-throughput, quantitative cell-based screen for efficient tailoring of RNA device activity. Nucleic Acids Res. 40, e154 (2012).
Tinoco, I. & Bustamante, C. How RNA folds. J. Mol. Biol. 293, 271–281 (1999).
Mustoe, A.M., Brooks, C.L. & Al-Hashimi, H.M. Hierarchy of RNA functional dynamics. Annu. Rev. Biochem. 83, 441–466 (2014).
Khvorova, A., Lescoute, A., Westhof, E. & Jayasena, S.D. Sequence elements outside the hammerhead ribozyme catalytic core enable intracellular activity. Nat. Struct. Biol. 10, 708–712 (2003).
Beisel, C.L. & Smolke, C.D. Design principles for riboswitch function. PLoS Comput. Biol. 5, e1000363 (2009).
Perreault, J. et al. Identification of hammerhead ribozymes in all domains of life reveals novel structural variations. PLoS Comput. Biol. 7, e1002031 (2011).
Link, K.H. et al. Engineering high-speed allosteric hammerhead ribozymes. Biol. Chem. 388, 779–786 (2007).
Desai, S.K. & Gallivan, J.P. Genetic screens and selections for small molecules based on a synthetic riboswitch that activates protein translation. J. Am. Chem. Soc. 126, 13247–13254 (2004).
Fowler, C.C., Brown, E.D. & Li, Y. A FACS-based approach to engineering artificial riboswitches. ChemBioChem 9, 1906–1911 (2008).
Lynch, S.A. & Gallivan, J.P. A flow cytometry-based screen for synthetic riboswitches. Nucleic Acids Res. 37, 184–192 (2009).
Nomura, Y. & Yokobayashi, Y. Dual selection of a genetic switch by a single selection marker. Biosystems 90, 115–120 (2007).
Topp, S. & Gallivan, J.P. Random walks to synthetic riboswitches—a high-throughput selection based on cell motility. ChemBioChem 9, 210–213 (2008).
Noderer, W.L. et al. Quantitative analysis of mammalian translation initiation sites by FACS-seq. Mol. Syst. Biol. 10, 748 (2014).
Goodman, D.B., Church, G.M. & Kosuri, S. Causes and effects of N-terminal codon bias in bacterial genes. Science 342, 475–479 (2013).
Kinney, J.B., Murugan, A., Callan, C.G. & Cox, E.C. Using deep sequencing to characterize the biophysical mechanism of a transcriptional regulatory sequence. Proc. Natl. Acad. Sci. USA 107, 9158–9163 (2010).
Gertz, J., Siggia, E.D. & Cohen, B.A. Analysis of combinatorial cis-regulation in synthetic and genomic promoters. Nature 457, 215–218 (2009).
Raveh-Sadka, T. et al. Manipulating nucleosome disfavoring sequences allows fine-tune regulation of gene expression in yeast. Nat. Genet. 44, 743–750 (2012).
Shalem, O. et al. Measurements of the impact of 3′ end sequences on gene expression reveal wide range and sequence dependent effects. PLoS Comput. Biol. 9, e1002934 (2013).
Sharon, E. et al. Inferring gene regulatory logic from high-throughput measurements of thousands of systematically designed promoters. Nat. Biotechnol. 30, 521–530 (2012).
Patwardhan, R.P. et al. Massively parallel functional dissection of mammalian enhancers in vivo. Nat. Biotechnol. 30, 265–270 (2012).
Kosuri, S. et al. Composability of regulatory sequences controlling transcription and translation in Escherichia coli. Proc. Natl. Acad. Sci. USA 110, 14024–14029 (2013).
Zimmermann, G.R., Shields, T.P., Jenison, R.D., Wick, C.L. & Pardi, A. A semiconserved residue inhibits complex formation by stabilizing interactions in the free state of a theophylline-binding RNA. Biochemistry 37, 9186–9192 (1998).
Kennedy, A.B., Liang, J.C. & Smolke, C.D. A versatile cis-blocking and trans-activation strategy for ribozyme characterization. Nucleic Acids Res. 41, e41 (2013).
Redden, H., Morse, N. & Alper, H.S. The synthetic biology toolbox for tuning gene expression in yeast. FEMS Yeast Res. doi:10.1111/1567-1364.12188 (2015).
Weigand, J.E. et al. Screening for engineered neomycin riboswitches that control translation initiation. RNA 14, 89–97 (2008).
Berens, C., Thain, A. & Schroeder, R. A tetracycline-binding RNA aptamer. Bioorg. Med. Chem. 9, 2549–2556 (2001).
Leontis, N.B., Stombaugh, J. & Westhof, E. The non-Watson-Crick base pairs and their associated isostericity matrices. Nucleic Acids Res. 30, 3497–3531 (2002).
Jenison, R.D., Gill, S.C., Pardi, A. & Polisky, B. High-resolution molecular discrimination by RNA. Science 263, 1425–1429 (1994).
Chao, G. et al. Isolating and engineering human antibodies using yeast surface display. Nat. Protoc. 1, 755–768 (2006).
Krueger, F., Andrews, S.R. & Osborne, C.S. Large scale loss of data in low-diversity Illumina sequencing libraries can be recovered by deferred cluster calling. PLoS ONE 6, e16607 (2011).
Zhang, J., Kobert, K., Flouri, T. & Stamatakis, A. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30, 614–620 (2014).
Milo, R. & Phillips, R. in Cell Biology by the Numbers http://book.bionumbers.org/how-much-cell-to-cell-variability-exists-in-protein-expression/ (2014).
Gietz, R.D. & Schiestl, R.H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2, 38–41 (2007).
Myszka, D.G. Improving biosensor analysis. J. Mol. Recognit. 12, 279–284 (1999).
Chang, A.L., McKeague, M., Liang, J.C. & Smolke, C.D. Kinetic and equilibrium binding characterization of aptamers to small molecules using a label-free, sensitive, and scalable platform. Anal. Chem. 86, 3273–3278 (2014).
Berman, H.M. et al. The Protein Data Bank. Nucleic Acids Res. 28, 235–242 (2000).
Chi, Y.-I. et al. Capturing hammerhead ribozyme structures in action by modulating general base catalysis. PLoS Biol. 6, e234 (2008).
Clore, G.M. & Kuszewski, J. Improving the accuracy of NMR structures of RNA by means of conformational database potentials of mean force as assessed by complete dipolar coupling cross-validation. J. Am. Chem. Soc. 125, 1518–1525 (2003).
Sarver, M., Zirbel, C.L., Stombaugh, J., Mokdad, A. & Leontis, N.B. FR3D: Finding local and composite recurrent structural motifs in RNA 3D structures. J. Math. Biol. 56, 215–252 (2008).
Acknowledgements
We thank M. McKeague and C. Schmidt for valuable feedback in the preparation of this manuscript; and C. Crumpton, M. Bigos and B. Gomez of the Stanford Shared FACS facility. This work was supported by funds from the US National Institutes of Health (grants GM086663, AT007886 to C.D.S. and Shared Instrumentation Grant S10RR025518-01), Defense Advanced Research Projects Agency (grant HR0011-11-2-0002 to C.D.S.), the Human Frontiers Science Program (grant RGP0054/2013 to C.D.S.), Natural Sciences and Engineering Research Council of Canada (fellowship to A.B.K.) and Agency for Science, Technology and Research (fellowship to J.S.X.).
Author information
Authors and Affiliations
Contributions
A.B.K. and B.T. have contributed equally to this work. The author order was chosen at random. A.B.K., B.T. and C.D.S. conceived the project and wrote the manuscript. A.B.K., B.T. and J.S.X. conducted the experiments. B.T. developed the software to analyze the cytometry and NGS data. B.T., A.B.K., J.S.X. and C.D.S. designed the experiments and analyzed the results.
Corresponding author
Ethics declarations
Competing interests
A.B.K. and C.D.S. are named on a pending patent application relating to RNA devices with ligand-responsive tertiary interactions (US application serial no. 62/186,767).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–22, Supplementary Tables 1–9 and Supplementary Notes 1 and 2 (PDF 7216 kb)
Supplementary Data 1
Supplementary Figure 2 source data (XLSX 620 kb)
Supplementary Data 2
Supplementary Figure 3 source data (XLSX 99 kb)
Supplementary Data 3
Supplementary Figure 4 source data (XLSX 42 kb)
Supplementary Data 4
Supplementary Figure 5 source data (XLSX 49 kb)
Supplementary Data 5
Supplementary Figure 6 source data (XLSX 56 kb)
Supplementary Data 6
Supplementary Figure 7 source data (XLSX 295 kb)
Supplementary Data 7
Supplementary Figure 8 source data (XLSX 76 kb)
Supplementary Data 8
Supplementary Figure 10 source data (XLSX 399 kb)
Supplementary Data 9
Supplementary Figure 11 source data (XLSX 400 kb)
Supplementary Data 10
Supplementary Figure 12 source data (XLSX 336 kb)
Supplementary Data 11
Supplementary Figure 13 source data (XLSX 310 kb)
Supplementary Data 12
Supplementary Figure 14 source data (XLSX 155 kb)
Supplementary Data 13
Supplementary Figure 15 source data (XLSX 164 kb)
Supplementary Data 14
Supplementary Figure 16 source data (XLSX 126 kb)
Supplementary Data 15
Supplementary Figure 17 source data (XLSX 301 kb)
Supplementary Data 16
Supplementary Figure 18 source data (XLSX 49 kb)
Rights and permissions
About this article
Cite this article
Townshend, B., Kennedy, A., Xiang, J. et al. High-throughput cellular RNA device engineering. Nat Methods 12, 989–994 (2015). https://doi.org/10.1038/nmeth.3486
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3486
This article is cited by
-
Deep generative design of RNA family sequences
Nature Methods (2024)
-
Genome-wide promoter responses to CRISPR perturbations of regulators reveal regulatory networks in Escherichia coli
Nature Communications (2023)
-
Engineering synthetic RNA devices for cell control
Nature Reviews Genetics (2022)
-
A multiplexed, automated evolution pipeline enables scalable discovery and characterization of biosensors
Nature Communications (2021)
-
High-throughput identification of synthetic riboswitches by barcode-free amplicon-sequencing in human cells
Nature Communications (2020)