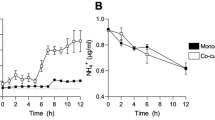
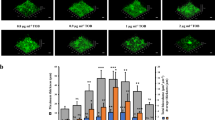
Abstract
Virulence of pathogenic bacteria is a tightly controlled process to facilitate invasion and survival in host tissues. Although pathways controlling virulence have been defined in detail, signals modulating these processes are poorly understood. The opportunistic pathogen Pseudomonas aeruginosa causes acute and chronic infections in humans. Disease progression is typically associated with a loss of acute virulence and the emergence of biofilms and chronic behaviour. The acute-to-chronic switch is governed by the global Gac/Rsm pathway. Using a newly developed acute–chronic dual reporter system we show that calcium stimulates the Gac/Rsm pathway via the Gac-associated hybrid histidine kinase LadS. We show that calcium binds to the periplasmic DISMED2 sensor domain of LadS to activate its kinase activity. Activation of the Gac/Rsm pathway by calcium leads to a switch to the chronic program and confers drug tolerance by reducing P. aeruginosa growth rate. Clinical isolates from cystic fibrosis airways retain their calcium response during chronic infections. Our data imply that calcium sensing evolved as an adaptation to the opportunistic lifestyle of P. aeruginosa and that calcium serves as a host signal to balance acute-to-chronic behaviour during infections. Establishing calcium signalling in host–pathogen interaction adds to growing evidence indicating key roles for calcium in bacterial signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Vakulskas, C. A., Potts, A. H., Babitzke, P. & Ahmer, B. M. M. Regulation of bacterial virulence by Csr (Rsm) systems. Microbiol. Mol. Biol. Rev. 79, 193–224 (2015).
Gonzalez Chavez, R., Alvarez, A. F., Romeo, T. & Georgellis, D. The physiological stimulus for the barA sensor kinase. J. Bacteriol. 192, 2009–2012 (2010).
Lawhon, S. D., Maurer, R., Suyemoto, M. & Altier, C. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol. Microbiol. 46, 1451–1464 (2002).
LeRoux, M. et al. Kin cell lysis is a danger signal that activates antibacterial pathways of Pseudomonas aeruginosa. eLife 4, e05701 (2015).
Heeb, S., Blumer, C. & Haas, D. Regulatory RNA as mediator in gacA/rsmA-dependent global control of exoproduct formation in Pseudomonas regulatory RNA as mediator in gacA/rsmA-dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J. Bacteriol. 184, 1046–1056 (2002).
Cystic Fibrosis Foundation Patient Registry 2013 Annual Data Report to the Center Directors (Cystic Fibrosis Foundation, 2014); https://www.cff.org/2013_CFF_Annual_Data_Report_to_the_Center_Directors.pdf
Cullen, L. & McClean, S. Bacterial adaptation during chronic respiratory infections. Pathogens 4, 66–89 (2015).
Sousa, A. & Pereira, M. Pseudomonas aeruginosa diversification during infection development in cystic fibrosis lungs—a review. Pathogens 3, 680–703 (2014).
Goodman, A. L. et al. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell 7, 745–754 (2004).
Goodman, A. L. et al. Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev. 23, 249–259 (2009).
Brencic, A. & Lory, S. Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA. Mol. Microbiol. 72, 612–632 (2009).
Kay, E. et al. Two gacA-dependent small RNAs modulate the quorum-sensing response in Pseudomonas aeruginosa. J. Bacteriol. 188, 6026–6033 (2006).
Brencic, A. et al. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol. Microbiol. 73, 434–445 (2009).
Ventre, I. et al. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc. Natl Acad. Sci. USA 103, 171–176 (2006).
Yahr, T. L. & Wolfgang, M. C. Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 62, 631–640 (2006).
Dasgupta, N., Ashare, A., Hunninghake, G. W. & Yahr, T. L. Transcriptional induction of the Pseudomonas aeruginosa type III secretion system by low Ca2+ and host cell contact proceeds through two distinct signaling pathways. Infect. Immun. 74, 3334–3341 (2006).
Mccaw, M. L. et al. Exsd is a negative regulator of the Pseudomonas aeruginosa type III secretion regulon. Mol. Microbiol. 46, 1123–1133 (2002).
Mulcahy, H., O'Callaghan, J., O'Grady, E. P., Adams, C. & O'Gara, F. The posttranscriptional regulator RsmA plays a role in the interaction between Pseudomonas aeruginosa and human airway epithelial cells by positively regulating the type III secretion system. Infect. Immun. 74, 3012–3015 (2006).
Zuber, S. et al. Gacs sensor domains pertinent to the regulation of exoproduct formation and to the biocontrol potential of Pseudomonas fluorescens CHA0. Mol. Plant Microbe Interact. 16, 634–644 (2003).
Kong, W. et al. Hybrid sensor kinase PA1611 in Pseudomonas aeruginosa regulates transitions between acute and chronic infection through direct interaction with RetS. Mol. Microbiol. 88, 784–797 (2013).
Groisman, E. A. The pleiotropic two-component regulatory system phoP-PhoQ. J. Bacteriol. 183, 1835–1842 (2001).
Sarkisova, S. A. et al. A Pseudomonas aeruginosa EF-Hand protein, efhP (PA4107), modulates stress responses and virulence at high calcium concentration. PLoS ONE 9, e98985 (2014).
Mikkelsen, H., McMullan, R. & Filloux, A. The Pseudomonas aeruginosa reference strain PA14 displays increased virulence due to a mutation in ladS. PLoS ONE 6, e29113 (2011).
Rigden, D. J. & Galperin, M. Y. The DxDxDG motif for calcium binding: multiple structural contexts and implications for evolution. J. Mol. Biol. 343, 971–984 (2004).
Basu Roy, A. & Sauer, K. Diguanylate cyclase NicD-based signalling mechanism of nutrient-induced dispersion by Pseudomonas aeruginosa. Mol. Microbiol. 94, 771–793 (2014).
Rybtke, M. T. et al. Fluorescence-based reporter for gauging cyclic di-GMP levels in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 78, 5060–5069 (2012).
Heeb, S. & Haas, D. Regulatory roles of the gacS/GacA two-component system in plant-associated and other Gram-negative bacteria. Mol. Plant Microbe Interact. 14, 1351–1363 (2001).
Burrowes, E., Baysse, C., Adams, C. & O'Gara, F. Influence of the regulatory protein RsmA on cellular functions in Pseudomonas aeruginosa PAO1, as revealed by transcriptome analysis. Microbiology 152, 405–418 (2006).
Wolfgang, M. C., Lee, V. T., Gilmore, M. E. & Lory, S. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell 4, 253–263 (2003).
Claudi, B. et al. Phenotypic variation of Salmonella in host tissues delays eradication by antimicrobial chemotherapy. Cell 158, 722–733 (2014).
Helaine, S. et al. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 343, 204–208 (2014).
Wotman, S., Mandel, I. D., Mercadante, J. & Denning, C. R. Parotid and submaxillary calcium in human cystic fibrosis. Arch. Oral Biol. 16, 663–665 (1971).
Shapiro, B. L. & Lam, L. F. Calcium and age in fibroblasts from control subjects and patients with cystic fibrosis. Science 216, 417–419 (1982).
von Ruecker, A. A., Bertele, R. & Harms, H. K. Calcium metabolism and cystic fibrosis: mitochondrial abnormalities suggest a modification of the mitochondrial membrane. Pediatr. Res. 18, 594–599 (1984).
Smith, E. E. et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl Acad. Sci. USA 103, 8487–8492 (2006).
Jain, M. et al. Type III secretion phenotypes of Pseudomonas aeruginosa strains change during infection of individuals with cystic fibrosis. J. Clin. Microbiol. 42, 5229–5237 (2004).
Vincent, F. et al. Distinct oligomeric forms of the Pseudomonas aeruginosa RetS sensor domain modulate accessibility to the ligand binding site. Environ. Microbiol. 12, 1775–1786 (2010).
Cioci, G. et al. Structural basis of calcium and galactose recognition by the lectin PA-IL of Pseudomonas aeruginosa. FEBS Lett. 555, 297–301 (2003).
Suzuki, T., Takagi, T., Furukohri, T., Kawamura, K. & Nakauchi, M. A calcium-dependent galactose-binding lectin from the tunicate Polyandrocarpa misakiensis. Isolation, characterization, and amino acid sequence. J. Biol. Chem. 265, 1274–1281 (1990).
Jamal-Talabani, S. et al. Ab initio structure determination and functional characterization of CBM36. Structure 12, 1177–1187 (2004).
Bolam, D. N. et al. X4 modules represent a new family of carbohydrate-binding modules that display novel properties. J. Biol. Chem. 279, 22953–22963 (2004).
Loris, R., Tielker, D., Jaeger, K. & Wyns, L. Structural basis of carbohydrate recognition by the lectin lecB from Pseudomonas aeruginosa. J. Mol. Biol. 331, 861–870 (2003).
Berridge, M. J., Lipp, P. & Bootman, M. D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 (2000).
Jones, H. E., Holland, I. B. & Campbell, A. K. Direct measurement of free Ca2+ shows different regulation of Ca2+ between the periplasm and the cytosol of Escherichia coli. Cell Calcium 32, 183–192 (2002).
Guragain, M., Lenaburg, D. L., Moore, F. S., Reutlinger, I. & Patrauchan, M. A. Calcium homeostasis in Pseudomonas aeruginosa requires multiple transporters and modulates swarming motility. Cell Calcium 54, 350–361 (2013).
Onek, L. A. & Smith, R. J. Calmodulin and calcium mediated regulation in prokaryotes. J. Gen. Microbiol. 138, 1039–1049 (1992).
Fry, I. J., Becker-Hapak, M. & Hageman, J. H. Purification and properties of an intracellular calmodulinlike protein from Bacillus subtilis cells. J. Bacteriol. 173, 2506–2513 (1991).
O'Hara, M. B. & Hageman, J. H. Energy and calcium ion dependence of proteolysis during sporulation of Bacillus subtilis cells. J. Bacteriol. 172, 4161–4170 (1990).
Inouye, S., Franceschini, T. & Inouye, M. Structural similarities between the development-specific protein S from a Gram-negative bacterium, Myxococcus xanthus, and calmodulin. Proc. Natl Acad. Sci. USA 80, 6829–6833 (1983).
Lee, V. T., Mazmanian, S. K. & Schneewind, O. A program of Yersinia enterocolitica type III secretion reactions is activated by specific signals. J. Bacteriol. 183, 4970–4978 (2001).
Frank, D. W. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26, 621–629 (1997).
Morgan, A. F. Transduction of Pseudomonas aeruginosa with a mutant of bacteriophage E79. J. Bacteriol. 139, 137–140 (1979).
Malone, J. G. et al. YfiBNR mediates cyclic di-GMP dependent small colony variant formation and persistence in Pseudomonas aeruginosa. PLoS Pathog. 6, e1000804 (2010).
Choi, K.-H. & Schweizer, H. P. An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol. 5, 30 (2005).
Voisard, C. et al. in Molecular Ecology of Rhizosphere Microorganisms (eds O'Gara, F., Dowling, D. & Boesten, B. ) 69–89 (Wiley, 1994).
Thanbichler, M., Iniesta, A. A. & Shapiro, L. A comprehensive set of plasmids for vanillate- and xylose-inducible gene expression in Caulobacter crescentus. Nucleic Acids Res. 35, e137 (2007).
Deuschle, K. et al. Rapid metabolism of glucose detected with FRET glucose nanosensors in epidermal cells and intact roots of Arabidopsis RNA-silencing mutants. Plant Cell 18, 2314–2325 (2006).
Radhakrishnan, S. K., Thanbichler, M. & Viollier, P. H. The dynamic interplay between a cell fate determinant and a lysozyme homolog drives the asymmetric division cycle of Caulobacter crescentus. Genes Dev. 22, 212–225 (2008).
Choi, K.-H. et al. A Tn7-based broad-range bacterial cloning and expression system. Nat. Methods 2, 443–448 (2005).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Glatter, T. et al. Large-scale quantitative assessment of different in-solution protein digestion protocols reveals superior cleavage efficiency of tandem Lys-C/trypsin proteolysis over trypsin digestion. J. Proteome Res. 11, 5145–5156 (2012).
Wiegand, I., Hilpert, K. & Hancock, R. E. W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3, 163–175 (2008).
Acknowledgements
The authors thank P. Manfredi for help with sequence analysis, B. Laventie for help with manuscript editing, J. Malone for bacterial strains and I. Attrée for the mini-CTX-PilV-GFP plasmid. This work was supported by a Werner Siemens PhD Fellowship to U.N.B. and by Swiss National Science Foundation grant 310030B_147090 to U.J.
Author information
Authors and Affiliations
Contributions
U.N.B. and U.J. designed the study, performed the analyses and wrote the paper. U.N.B. and T.J. collected and processed data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary Figures 1–11, Supplementary Table 1, legends for Supplementary Videos 1 and 2, Supplementary References (PDF 14697 kb)
Supplementary Video 1
Shift from high calcium to low calcium conditions (AVI 7688 kb)
Supplementary Video 2
Shift from low calcium to high calcium conditions (AVI 6779 kb)
Rights and permissions
About this article
Cite this article
Broder, U., Jaeger, T. & Jenal, U. LadS is a calcium-responsive kinase that induces acute-to-chronic virulence switch in Pseudomonas aeruginosa. Nat Microbiol 2, 16184 (2017). https://doi.org/10.1038/nmicrobiol.2016.184
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2016.184
This article is cited by
-
A Pseudomonas aeruginosa small RNA regulates chronic and acute infection
Nature (2023)
-
A genetic switch controls Pseudomonas aeruginosa surface colonization
Nature Microbiology (2023)
-
Mycobacterium abscessus pathogenesis identified by phenogenomic analyses
Nature Microbiology (2022)
-
EF-hand protein, EfhP, specifically binds Ca2+ and mediates Ca2+ regulation of virulence in a human pathogen Pseudomonas aeruginosa
Scientific Reports (2022)
-
Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics
Signal Transduction and Targeted Therapy (2022)