Abstract
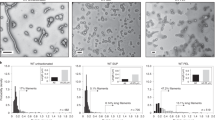
Many animal viruses are enveloped in a lipid bilayer taken up from cellular membranes. Because viral surface proteins bind to these membranes to initiate infection, we hypothesized that free virions may also be capable of interacting with the envelopes of other virions extracellularly. Here, we demonstrate this hypothesis in the vesicular stomatitis virus (VSV), a prototypic negative-strand RNA virus composed of an internal ribonucleocapsid, a matrix protein and an external envelope1. Using microscopy, dynamic light scattering, differential centrifugation and flow cytometry, we show that free viral particles can spontaneously aggregate into multi-virion infectious units. We also show that, following establishment of these contacts, different viral genetic variants are co-transmitted to the same target cell. Furthermore, virion–virion binding can determine key aspects of viral fitness such as antibody escape. In purified virions, this process is driven by protein–lipid interactions probably involving the VSV surface glycoprotein and phosphatidylserine. Whereas we found that multi-virion complexes occurred unfrequently in standard cell cultures, they were abundant in other fluids such as saliva, a natural VSV shedding route2. Our findings contrast with the commonly accepted perception of virions as passive propagules and show the ability of enveloped viruses to establish collective infectious units, which could in turn facilitate the evolution of virus–virus interactions and of social-like traits3.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ge, P. et al. Cryo-EM model of the bullet-shaped vesicular stomatitis virus. Science 327, 689–693 (2010).
Letchworth, G. J., Rodríguez, L. L. & Del Cbarrera, J. Vesicular stomatitis. Vet. J. 157, 239–260 (1999).
Sanjuán, R. Collective infectious units in viruses. Trends Microbiol. 25, 402–412 (2017).
Sanjuán, R., Moya, A. & Elena, S. F. The distribution of fitness effects caused by single-nucleotide substitutions in an RNA virus. Proc. Natl Acad. Sci. USA 101, 8396–8401 (2004).
Kalvodova, L. et al. The lipidomes of vesicular stomatitis virus, Semliki Forest virus, and the host plasma membrane analyzed by quantitative shotgun mass spectrometry. J. Virol. 83, 7996–8003 (2009).
Coil, D. A. & Miller, A. D. Phosphatidylserine is not the cell surface receptor for vesicular stomatitis virus. J. Virol. 78, 10920–10926 (2004).
Carneiro, F. A. et al. Probing the interaction between vesicular stomatitis virus and phosphatidylserine. Eur. Biophys. J. 35, 145–154 (2006).
Hall, M. P., Burson, K. K. & Huestis, W. H. Interactions of a vesicular stomatitis virus G protein fragment with phosphatidylserine: NMR and fluorescence studies. Biochim. Biophys. Acta 1415, 101–113 (1998).
MacFarlane, M. G. The biochemistry of bacterial toxins; the enzymic specificity of Clostridium welchii lecithinase. Biochem. J. 42, 587–590 (1948).
Tait, J. F. & Gibson, D. Phospholipid binding of annexin V: effects of calcium and membrane phosphatidylserine content. Arch. Biochem. Biophys. 298, 187–191 (1992).
Moerdyk-Schauwecker, M., Hwang, S. I. & Grdzelishvili, V. Z. Cellular proteins associated with the interior and exterior of vesicular stomatitis virus virions. PLoS ONE 9, e104688 (2014).
Finkelshtein, D., Werman, A., Novick, D., Barak, S. & Rubinstein, M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl Acad. Sci. USA 110, 7306–7311 (2013).
Prasad, J. M., Migliorini, M., Galisteo, R. & Strickland, D. K. Generation of a potent low density lipoprotein receptor-related protein 1 (LRP1) antagonist by engineering a stable form of the receptor-associated protein (RAP) D3 domain. J. Biol. Chem. 290, 17262–17268 (2015).
Simon, K. O., Cardamone, J. J. Jr, Whitaker-Dowling, P. A., Youngner, J. S. & Widnell, C. C. Cellular mechanisms in the superinfection exclusion of vesicular stomatitis virus. Virology 177, 375–379 (1990).
Bald, J. G. & Briggs, G. E. Aggregation of virus particles. Nature 140, 111 (1937).
Galasso, G. J. & Sharp, D. G. Virus particle aggregation and the plaque-forming unit. J. Immunol. 88, 339–347 (1962).
Wallis, C. & Melnick, J. L. Virus aggregation as the cause of the non-neutralizable persistent fraction. J. Virol. 1, 478–488 (1967).
Galasso, G. J. Quantitative studies on the quality, effects of aggregation and thermal inactivation of vesicular stomatitis virus. Arch. Gesamte Virusforsch. 21, 437–446 (1967).
Floyd, R. Viral aggregation: mixed suspensions of poliovirus and reovirus. Appl. Environ. Microbiol. 38, 980–986 (1979).
Combe, M., Garijo, R., Geller, R., Cuevas, J. M. & Sanjuán, R. Single-cell analysis of RNA virus infection identifies multiple genetically diverse viral genomes within single infectious units. Cell Host Microbe 18, 424–432 (2015).
Aguilera, E. R., Erickson, A. K., Jesudhasan, P. R., Robinson, C. M. & Pfeiffer, J. K. Plaques formed by mutagenized viral populations have elevated coinfection frequencies. mBio 8, e02020–16 (2017).
Marcus, P. I., Rodríguez, L. L. & Sekellick, M. J. Interferon induction as a quasispecies marker of vesicular stomatitis virus populations. J. Virol. 72, 542–549 (1998).
Nunamaker, R. A. et al. Grasshoppers (Orthoptera: Acrididae) could serve as reservoirs and vectors of vesicular stomatitis virus. J. Med. Entomol. 40, 957–963 (2003).
Scherer, C. F. et al. Vesicular stomatitis New Jersey virus (VSNJV) infects keratinocytes and is restricted to lesion sites and local lymph nodes in the bovine, a natural host. Vet. Res. 38, 375–390 (2007).
Smith, P. F. et al. Mechanical transmission of vesicular stomatitis New Jersey virus by Simulium vittatum (Diptera: Simuliidae) to domestic swine (Sus scrofa). J. Med. Entomol. 46, 1537–1540 (2009).
Smith, P. F. et al. Host predilection and transmissibility of vesicular stomatitis New Jersey virus strains in domestic cattle (Bos taurus) and swine (Sus scrofa). BMC Vet. Res. 8, 183–188 (2012).
Libersou, S. et al. Distinct structural rearrangements of the VSV glycoprotein drive membrane fusion. J. Cell Biol. 191, 199–210 (2010).
Nagata, S., Hanayama, R. & Kawane, K. Autoimmunity and the clearance of dead cells. Cell 140, 619–630 (2010).
Amara, A. & Mercer, J. Viral apoptotic mimicry. Nat. Rev. Microbiol. 13, 461–469 (2015).
Chen, Y. H. et al. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell 160, 619–630 (2015).
Gutiérrez, S., Michalakis, Y. & Blanc, S. Virus population bottlenecks during within-host progression and host-to-host transmission. Curr. Opin. Virol. 2, 546–555 (2012).
Aaskov, J., Buzacott, K., Thu, H. M., Lowry, K. & Holmes, E. C. Long-term transmission of defective RNA viruses in humans and Aedes mosquitoes. Science 311, 236–238 (2006).
Stack, J. C., Murcia, P. R., Grenfell, B. T., Wood, J. L. & Holmes, E. C. Inferring the inter-host transmission of influenza A virus using patterns of intra-host genetic variation. Proc. Biol. Sci. 280, 20122173 (2013).
Marriott, A. C. & Dimmock, N. J. Defective interfering viruses and their potential as antiviral agents. Rev. Med. Virol. 20, 51–62 (2010).
Notton, T., Sardanyes, J., Weinberger, A. D. & Weinberger, L. S. The case for transmissible antivirals to control population-wide infectious disease. Trends Biotechnol. 32, 400–405 (2014).
Vandepol, S. B., Lefrancois, L. & Holland, J. J. Sequences of the major antibody binding epitopes of the Indiana serotype of vesicular stomatitis virus. Virology 148, 312–325 (1986).
Whelan, S. P., Ball, L. A., Barr, J. N. & Wertz, G. T. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc. Natl. Acad. Sci. USA 92, 8388–8392 (1995).
Hassan, P. A., Rana, S. & Verma, G. Making sense of Brownian motion: colloid characterization by dynamic light scattering. Langmuir 31, 3–12 (2015).
Acknowledgements
The authors thank D. Vie, A. Flores, R. Garijo and S. Torres for technical assistance, and personnel from La Muntanyeta Coop. V. for providing access to cows. This work was financially supported by grants from the European Research Council (ERC-2011-StG- 281191-VIRMUT and ERC-2016-CoG-724519-Vis-à-Vis) and the Spanish MINECO (BFU2013-41329) to R.S. and by a Ramón y Cajal contract to J.M.C.
Author information
Authors and Affiliations
Contributions
M.D.-M. and J.M.C contributed equally to this work and performed all experiments. R.S. conceived the study, supervised the experiments, analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–4, Supplementary Table 2. (PDF 503 kb)
Supplementary Table 1
Flow cytometry data used in co-fluorescence tests. (XLSX 21 kb)
Supplementary Video 1
NTA of purified VSV virions untreated. Each dot is the light scattered by an individual VSV particle. NTA uses Brownian motion to infer particle size. Particles flow from left to right because they were pumped by a syringe. This translation movement is discarded for particle size inference. Light intensity increases with particle size, but depends also on the position of the particles in the diluent column (distance to lens) and hence is not used for size inference. (AVI 412 kb)
Supplementary Video 2
NTA of purified VSV virions incubated 1 h at 37 °C. Each dot is the light scattered by an individual VSV particle. NTA uses Brownian motion to infer particle size. Particles flow from left to right because they were pumped by a syringe. This translation movement is discarded for particle size inference. Light intensity increases with particle size, but depends also on the position of the particles in the diluent column (distance to lens) and hence is not used for size inference. (AVI 592 kb)
Rights and permissions
About this article
Cite this article
Cuevas, J., Durán-Moreno, M. & Sanjuán, R. Multi-virion infectious units arise from free viral particles in an enveloped virus. Nat Microbiol 2, 17078 (2017). https://doi.org/10.1038/nmicrobiol.2017.78
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2017.78
This article is cited by
-
Genetic complementation fosters evolvability in complex fitness landscapes
Scientific Reports (2023)
-
Ten recent insights for our understanding of cooperation
Nature Ecology & Evolution (2021)
-
Viral and host heterogeneity and their effects on the viral life cycle
Nature Reviews Microbiology (2021)
-
Defective viral genomes are key drivers of the virus–host interaction
Nature Microbiology (2019)