Abstract
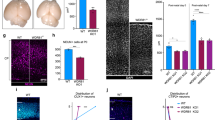
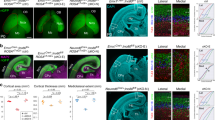
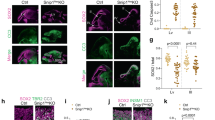
Brain structure and size require precise division of neural stem cells (NSCs), which self-renew and generate intermediate neural progenitors (INPs) and neurons. The factors that regulate NSCs remain poorly understood, and mechanistic explanations of how aberrant NSC division causes the reduced brain size seen in microcephaly are lacking. Here we show that Magoh, a component of the exon junction complex (EJC) that binds RNA, controls mouse cerebral cortical size by regulating NSC division. Magoh haploinsufficiency causes microcephaly because of INP depletion and neuronal apoptosis. Defective mitosis underlies these phenotypes, as depletion of EJC components disrupts mitotic spindle orientation and integrity, chromosome number and genomic stability. In utero rescue experiments showed that a key function of Magoh is to control levels of the microcephaly-associated protein Lis1 during neurogenesis. Our results uncover requirements for the EJC in brain development, NSC maintenance and mitosis, thereby implicating this complex in the pathogenesis of microcephaly.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
Change history
19 April 2010
In the version of this article initially published online, the last sentence of the sixth paragraph in the Discussion section read “Future studies will reveal, for example, whether any of the genes altered in our microarray or proteomics experiments are also essential targets of MAGOH.” MAGOH has been corrected to Magoh, denoting the mouse gene. Also, the second sentence of the last paragraph in the Discussion section initially read “Of note, Magoh is found within a 55-gene deletion on chromosome 1p32.3 that is associated with mental retardation and abnormalities in brain size31.” Magoh has been corrected to MAGOH, denoting the human gene. These errors have been corrected in the print, HTML and PDF versions of the article.
References
Molyneaux, B.J., Arlotta, P., Menezes, J.R. & Macklis, J.D. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 8, 427–437 (2007).
Haubensak, W., Attardo, A., Denk, W. & Huttner, W.B. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl. Acad. Sci. USA 101, 3196–201 (2004).
Pontious, A., Kowalczyk, T., Englund, C. & Hevner, R.F. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci 30, 24–32 (2008).
Chenn, A. & McConnell, S.K. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell 82, 631–641 (1995).
Sanada, K. & Tsai, L.H. G protein βγ subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell 122, 119–131 (2005).
Zhong, W., Feder, J.N., Jiang, M.M., Jan, L.Y. & Jan, Y.N. Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron 17, 43–53 (1996).
Bond, J. & Woods, C.G. Cytoskeletal genes regulating brain size. Curr. Opin. Cell Biol. 18, 95–101 (2006).
Bond, J. et al. ASPM is a major determinant of cerebral cortical size. Nat. Genet. 32, 316–320 (2002).
Bond, J. et al. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat. Genet. 37, 353–355 (2005).
Jackson, A.P. et al. Identification of microcephalin, a protein implicated in determining the size of the human brain. Am. J. Hum. Genet. 71, 136–142 (2002).
do Carmo Avides, M. & Glover, D.M. Abnormal spindle protein, Asp, and the integrity of mitotic centrosomal microtubule organizing centers. Science 283, 1733–1735 (1999).
Bi, W. et al. Increased LIS1 expression affects human and mouse brain development. Nat. Genet. 41, 168–177 (2009).
Faulkner, N.E. et al. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat. Cell Biol. 2, 784–791 (2000).
Reiner, O. et al. Isolation of a Miller-Dieker lissencephaly gene containing G protein β-subunit-like repeats. Nature 364, 717–721 (1993).
Griffith, E. et al. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat. Genet. 40, 232–236 (2008).
O'Driscoll, M., Ruiz-Perez, V.L., Woods, C.G., Jeggo, P.A. & Goodship, J.A. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat. Genet. 33, 497–501 (2003).
Rauch, A. et al. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science 319, 816–819 (2008).
Matera, I. et al. A sensitized mutagenesis screen identifies Gli3 as a modifier of Sox10 neurocristopathy. Hum. Mol. Genet. 17, 2118–2131 (2008).
Diem, M.D., Chan, C.C., Younis, I. & Dreyfuss, G. PYM binds the cytoplasmic exon-junction complex and ribosomes to enhance translation of spliced mRNAs. Nat. Struct. Mol. Biol. 14, 1173–1179 (2007).
Kataoka, N., Diem, M.D., Kim, V.N., Yong, J. & Dreyfuss, G. Magoh, a human homolog of Drosophila mago nashi protein, is a component of the splicing-dependent exon-exon junction complex. EMBO J. 20, 6424–6433 (2001).
Le Hir, H., Gatfield, D., Braun, I.C., Forler, D. & Izaurralde, E. The protein Mago provides a link between splicing and mRNA localization. EMBO Rep. 2, 1119–1124 (2001).
Nott, A., Le Hir, H. & Moore, M.J. Splicing enhances translation in mammalian cells: an additional function of the exon junction complex. Genes Dev. 18, 210–222 (2004).
Palacios, I.M., Gatfield, D., St. Johnston, D. & Izaurralde, E. An eIF4AIII-containing complex required for mRNA localization and nonsense-mediated mRNA decay. Nature 427, 753–757 (2004).
Pawlisz, A.S. et al. Lis1-Nde1-dependent neuronal fate control determines cerebral cortical size and lamination. Hum. Mol. Genet. 17, 2441–2455 (2008).
Gambello, M.J. et al. Multiple dose-dependent effects of Lis1 on cerebral cortical development. J. Neurosci. 23, 1719–1729 (2003).
Yingling, J. et al. Neuroepithelial stem cell proliferation requires Lis1 for precise spindle orientation and symmetric division. Cell 132, 474–486 (2008).
Micklem, D.R. et al. The mago nashi gene is required for the polarisation of the oocyte and the formation of perpendicular axes in Drosophila. Curr. Biol. 7, 468–478 (1997).
Azzalin, C.M. & Lingner, J. The human RNA surveillance factor UPF1 is required for S phase progression and genome stability. Curr. Biol. 16, 433–439 (2006).
van der Weele, C.M., Tsai, C.W. & Wolniak, S.M. Mago nashi is essential for spermatogenesis in Marsilea. Mol. Biol. Cell 18, 3711–3722 (2007).
Yamada, M. et al. Inhibition of calpain increases LIS1 expression and partially rescues in vivo phenotypes in a mouse model of lissencephaly. Nat. Med. 15, 1202–1207 (2009).
Mulatinho, M., Llerena, J., Leren, T.P., Rao, P.N. & Quintero-Rivera, F. Deletion (1)(p32.2-p32.3) detected by array-CGH in a patient with developmental delay/mental retardation, dysmorphic features and low cholesterol: A new microdeletion syndrome? Am. J. Med. Genet. A. 146A, 2284–2290 (2008).
Brunetti-Pierri, N. et al. Recurrent reciprocal 1q21.1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nat. Genet. 40, 1466–1471 (2008).
Mefford, H.C. et al. Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. N. Engl. J. Med. 359, 1685–1699 (2008).
Lundsteen, C. & Lind, A.M. A test of a climate room for preparation of chromosome slides. Clin. Genet. 28, 260–262 (1985).
Lichter, P., Cremer, T., Borden, J., Manuelidis, L. & Ward, D.C. Delineation of individual human chromosomes in metaphase and interphase cells by in situ suppression hybridization using recombinant DNA libraries. Hum. Genet. 80, 224–234 (1988).
Pinkel, D., Straume, T. & Gray, J.W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc. Natl. Acad. Sci. USA 83, 2934–2938 (1986).
Liyanage, M. et al. Multicolour spectral karyotyping of mouse chromosomes. Nat. Genet. 14, 312–315 (1996).
Schrock, E. et al. Multicolor spectral karyotyping of human chromosomes. Science 273, 494–497 (1996).
Gaiano, N., Nye, J.S. & Fishell, G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron 26, 395–404 (2000).
Kittler, R. et al. An endoribonuclease-prepared siRNA screen in human cells identifies genes essential for cell division. Nature 432, 1036–1040 (2004).
Visel, A., Thaller, C. & Eichele, G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 32, D552–D556 (2004).
Saito, T. In vivo electroporation in the embryonic mouse central nervous system. Nat. Protocols 1, 1552–1558 (2006).
Buac, K. et al. NRG1 / ERBB3 signaling in melanocyte development and melanoma: inhibition of differentiation and promotion of proliferation. Pigm. Cell Melanoma Res. 22, 773–784 (2009).
Acknowledgements
For advice, we thank Pavan laboratory members, including L. Baxter for reading the manuscript. For technical assistance, we thank A. Incao (mouse husbandry); G. Elliot and A. Chen (mouse transgenics); A. Dutra, E. Pak and S. Witchovitch (metaphase, SKY, microscopy assistance); S. Anderson and M. Kirby (FACS analysis); B. Bhorate (microarray statistics); M. Bryant (pathology analysis); Harvard Partners Center for Genetics and Genomics (candidate gene sequencing); and J. Fekecs and D. Leja (assistance with figures). This research was funded in part by an National Institute of General Medical Sciences PRAT fellowship and K99/R00 Pathway to Independence Award (to D.L.S.), by the Intramural Research program of NIH/NHGRI (to W.J.P., K.M.) and by the NIH/NHGRI (to N.G.). C.A.W. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
D.L.S. designed the study, performed all experiments except where noted otherwise and wrote the paper. D.E.W.-C. performed all mRNA analyses and assisted with quantitative analyses. K.C.S. and T.J.P. performed all in utero electroporations and dissections of electroporated brains. D.M.L. performed staining of E18.5 markers. A.J.B. assisted with HeLa cell analyses and quantification of NSCs and INPs. H.L. performed RPE cell analyses. D.L.S., W.J.P., C.A.W., N.G. and K.M. were involved in research design, and all authors were involved in data analysis. D.L.S. and W.J.P. prepared the manuscript. All authors have agreed to the content in the manuscript, including the data as presented.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1–3 (PDF 12447 kb)
Supplementary Data
Serum chemistries, hematology, and organ weights of control and mutant animals (XLS 83 kb)
Rights and permissions
About this article
Cite this article
Silver, D., Watkins-Chow, D., Schreck, K. et al. The exon junction complex component Magoh controls brain size by regulating neural stem cell division. Nat Neurosci 13, 551–558 (2010). https://doi.org/10.1038/nn.2527
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2527
This article is cited by
-
An entosis-like process induces mitotic disruption in Pals1 microcephaly pathogenesis
Nature Communications (2023)
-
Multifaceted roles of MAGOH Proteins
Molecular Biology Reports (2023)
-
Global ubiquitinome profiling identifies NEDD4 as a regulator of Profilin 1 and actin remodelling in neural crest cells
Nature Communications (2022)
-
Full function of exon junction complex factor, Rbm8a, is critical for interneuron development
Translational Psychiatry (2020)
-
Translating neural stem cells to neurons in the mammalian brain
Cell Death & Differentiation (2019)