Abstract
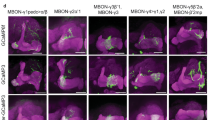
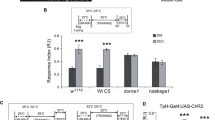
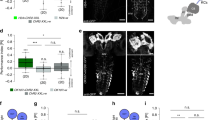
Aversive olfactory memory is formed in the mushroom bodies in Drosophila melanogaster. Memory retrieval requires mushroom body output, but the manner in which a memory trace in the mushroom body drives conditioned avoidance of a learned odor remains unknown. To identify neurons that are involved in olfactory memory retrieval, we performed an anatomical and functional screen of defined sets of mushroom body output neurons. We found that MB-V2 neurons were essential for retrieval of both short- and long-lasting memory, but not for memory formation or memory consolidation. MB-V2 neurons are cholinergic efferent neurons that project from the mushroom body vertical lobes to the middle superiormedial protocerebrum and the lateral horn. Notably, the odor response of MB-V2 neurons was modified after conditioning. As the lateral horn has been implicated in innate responses to repellent odorants, we propose that MB-V2 neurons recruit the olfactory pathway involved in innate odor avoidance during memory retrieval.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Keene, A.C. & Waddell, S. Drosophila olfactory memory: single genes to complex neural circuits. Nat. Rev. Neurosci. 8, 341–354 (2007).
McGuire, S.E., Deshazer, M. & Davis, R.L. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog. Neurobiol. 76, 328–347 (2005).
Tully, T., Preat, T., Boynton, S.C. & Del Vecchio, M. Genetic dissection of consolidated memory in Drosophila. Cell 79, 35–47 (1994).
Masse, N.Y., Turner, G.C. & Jefferis, G.S. Olfactory information processing in Drosophila. Curr. Biol. 19, R700–R713 (2009).
Aso, Y. et al. Specific dopaminergic neurons for the formation of labile aversive memory. Curr. Biol. 20, 1445–1451 (2010).
Claridge-Chang, A. et al. Writing memories with light-addressable reinforcement circuitry. Cell 139, 405–415 (2009).
Schwaerzel, M. et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10495–10502 (2003).
de Belle, J.S. & Heisenberg, M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 263, 692–695 (1994).
Wang, Y. et al. Blockade of neurotransmission in Drosophila mushroom bodies impairs odor attraction, but not repulsion. Curr. Biol. 13, 1900–1904 (2003).
Crittenden, J.R., Skoulakis, E.M., Han, K.A., Kalderon, D. & Davis, R.L. Tripartite mushroom body architecture revealed by antigenic markers. Learn. Mem. 5, 38–51 (1998).
Gervasi, N., Tchenio, P. & Preat, T. PKA dynamics in Drosophila olfactory learning and memory center: coincidence detection by Rutabaga adenylyl cyclase and regulation by Dunce phosphodiesterase. Neuron 65, 516–529 (2010).
Tomchik, S.M. & Davis, R.L. Dynamics of learning-related cAMP signaling and stimulus integration in the Drosophila olfactory pathway. Neuron 64, 510–521 (2009).
Wang, Y., Mamiya, A., Chiang, A.S. & Zhong, Y. Imaging of an early memory trace in the Drosophila mushroom body. J. Neurosci. 28, 4368–4376 (2008).
Yu, D., Akalal, D.B. & Davis, R.L. Drosophila alpha/beta mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron 52, 845–855 (2006).
Isabel, G., Pascual, A. & Preat, T. Exclusive consolidated memory phases in Drosophila. Science 304, 1024–1027 (2004).
McGuire, S.E., Le, P.T. & Davis, R.L. The role of Drosophila mushroom body signaling in olfactory memory. Science 293, 1330–1333 (2001).
Ito, K. et al. The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster Meigen. Learn. Mem. 5, 52–77 (1998).
Tanaka, N.K., Tanimoto, H. & Ito, K. Neuronal assemblies of the Drosophila mushroom body. J. Comp. Neurol. 508, 711–755 (2008).
Kitamoto, T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 47, 81–92 (2001).
Zhang, Y.Q., Rodesch, C.K. & Broadie, K. Living synaptic vesicle marker: synaptotagmin-GFP. Genesis 34, 142–145 (2002).
Robinson, I.M., Ranjan, R. & Schwarz, T.L. Synaptotagmins I and IV promote transmitter release independently of Ca2+ binding in the C(2)A domain. Nature 418, 336–340 (2002).
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999).
Krashes, M.J. & Waddell, S. Rapid consolidation to a radish and protein synthesis–dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J. Neurosci. 28, 3103–3113 (2008).
Colomb, J., Kaiser, L., Chabaud, M.A. & Preat, T. Parametric and genetic analysis of Drosophila appetitive long-term memory and sugar motivation. Genes Brain Behav. 8, 407–415 (2009).
Kitamoto, T. Conditional disruption of synaptic transmission induces male-male courtship behavior in Drosophila. Proc. Natl. Acad. Sci. USA 99, 13232–13237 (2002).
Sepp, K.J. & Auld, V.J. Conversion of lacZ enhancer trap lines to GAL4 lines using targeted transposition in Drosophila melanogaster. Genetics 151, 1093–1101 (1999).
Pfeiffer, B.D. et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. USA 105, 9715–9720 (2008).
Yasuyama, K., Meinertzhagen, I.A. & Schurmann, F.W. Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J. Comp. Neurol. 445, 211–226 (2002).
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881 (2009).
Mauelshagen, J. Neural correlates of olfactory learning paradigms in an identified neuron in the honeybee brain. J. Neurophysiol. 69, 609–625 (1993).
Okada, R., Rybak, J., Manz, G. & Menzel, R. Learning-related plasticity in PE1 and other mushroom body–extrinsic neurons in the honeybee brain. J. Neurosci. 27, 11736–11747 (2007).
Caporale, N. & Dan, Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu. Rev. Neurosci. 31, 25–46 (2008).
Bender, V.A., Bender, K.J., Brasier, D.J. & Feldman, D.E. Two coincidence detectors for spike timing–dependent plasticity in somatosensory cortex. J. Neurosci. 26, 4166–4177 (2006).
Hashimotodani, Y. et al. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron 45, 257–268 (2005).
Menzel, R. & Manz, G. Neural plasticity of mushroom body-extrinsic neurons in the honeybee brain. J. Exp. Biol. 208, 4317–4332 (2005).
Liu, X. & Davis, R.L. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat. Neurosci. 12, 53–59 (2009).
Bashir, Z.I. On long-term depression induced by activation of G protein–coupled receptors. Neurosci. Res. 45, 363–367 (2003).
Tanaka, N.K., Awasaki, T., Shimada, T. & Ito, K. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr. Biol. 14, 449–457 (2004).
Pascual, A. & Preat, T. Localization of long-term memory within the Drosophila mushroom body. Science 294, 1115–1117 (2001).
Tully, T. & Quinn, W.G. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J. Comp. Physiol. [A] 157, 263–277 (1985).
Wong, A.M., Wang, J.W. & Axel, R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell 109, 229–241 (2002).
Gordon, M.D. & Scott, K. Motor control in a Drosophila taste circuit. Neuron 61, 373–384 (2009).
Otsuna, H. & Ito, K. Systematic analysis of the visual projection neurons of Drosophila melanogaster. I. Lobula-specific pathways. J. Comp. Neurol. 497, 928–958 (2006).
Featherstone, D.E. et al. Presynaptic glutamic acid decarboxylase is required for induction of the postsynaptic receptor field at a glutamatergic synapse. Neuron 27, 71–84 (2000).
Sakai, T., Kasuya, J., Kitamoto, T. & Aigaki, T. The Drosophila TRPA channel, Painless, regulates sexual receptivity in virgin females. Genes Brain Behav. 8, 546–557 (2009).
Daniels, R.W., Gelfand, M.V., Collins, C.A. & DiAntonio, A. Visualizing glutamatergic cell bodies and synapses in Drosophila larval and adult CNS. J. Comp. Neurol. 508, 131–152 (2008).
Takagawa, K. & Salvaterra, P. Analysis of choline acetyltransferase protein in temperature sensitive mutant flies using newly generated monoclonal antibody. Neurosci. Res. 24, 237–243 (1996).
Neckameyer, W.S., Woodrome, S., Holt, B. & Mayer, A. Dopamine and senescence in Drosophila melanogaster. Neurobiol. Aging 21, 145–152 (2000).
Klagges, B.R. et al. Invertebrate synapsins: a single gene codes for several isoforms in Drosophila. J. Neurosci. 16, 3154–3165 (1996).
Fiala, A. & Spall, T. In vivo calcium imaging of brain activity in Drosophila by transgenic cameleon expression. Sci. STKE 2003, PL6 (2003).
Acknowledgements
We thank J. Urban and G.M. Technau for the MZ160 line, the members of the NP consortium for the NP2492 line, J.-M. Dura for the y1w1118;GAL80[y+],Sb line and for discussions, L.L. Looger for the UAS-GCaMP3 line, T. Kitamoto for the GAD1-GAL80 and Cha3.3kb-GAL80 lines, and V. Hakim, I. Rivals and members of our laboratories for discussions. This work was supported by grants from the Japan Society for the Promotion of Science (K.I.), the Agence Nationale pour la Recherche (T.P.), the Fondation Bettencourt-Schueler (T.P.), the Fondation pour la Recherche Médicale (T.P.), the Emmy-Noether Program from Deutsche Forschungsgemeinschaft (H.T.), the Bernstein Focus Learning from Bundesministerium für Bildung und Forschung (H.T.) and the Max-Planck-Gesellschaft (H.T.). J.S. and G.I. were supported by the Fondation pour la Recherche Médicale, P.-Y.P. was supported by a grant from Région Ile-de-France and Y.A. was supported by Deutscher Akademischer Austausch Dienst.
Author information
Authors and Affiliations
Contributions
Y.A. and H.T. designed and I.S., S.R.T. and V.T. carried out anatomical experiments. I.S., Y.A. and H.T. analyzed the microscopic data and assembled figures. K.I. and G.M.R. provided Gal4 lines with their expression patterns. J.S., G.I. and T.P. designed and J.S., S.T. and P.-Y.P. carried out behavior experiments. J.S. generated the NP2492-Gal80 mutant. P.-Y.P., T.P. and P.T. designed the in vivo imaging experiments and P.-Y.P. carried them out. J.S., P.-Y.P., I.S., Y.A., H.T. and T.P. wrote the paper. T.P. and H.T. supervised the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 16348 kb)
Supplementary Video 1
Expression pattern of NP2492 in the brain (MOV 3358 kb)
Supplementary Video 2
Expression pattern of MZ160 in the brain (MOV 2495 kb)
Supplementary Video 3
Expression pattern of MZ160, Cha3.3kb-GAL80 in the brain (MOV 1968 kb)
Supplementary Video 4
Expression pattern of MZ160, NP2492-GAL80 in the brain (MOV 2207 kb)
Supplementary Video 5
Expression pattern of R71D08 in the brain (MOV 2700 kb)
Rights and permissions
About this article
Cite this article
Séjourné, J., Plaçais, PY., Aso, Y. et al. Mushroom body efferent neurons responsible for aversive olfactory memory retrieval in Drosophila. Nat Neurosci 14, 903–910 (2011). https://doi.org/10.1038/nn.2846
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2846
This article is cited by
-
Forgotten memory storage and retrieval in Drosophila
Nature Communications (2023)
-
Assessing olfactory, memory, social and circadian phenotypes associated with schizophrenia in a genetic model based on Rim
Translational Psychiatry (2021)
-
Dopaminergic mechanism underlying reward-encoding of punishment omission during reversal learning in Drosophila
Nature Communications (2021)
-
Learning with reinforcement prediction errors in a model of the Drosophila mushroom body
Nature Communications (2021)
-
Olfactory processing in the lateral horn of Drosophila
Cell and Tissue Research (2021)