Abstract
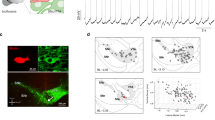
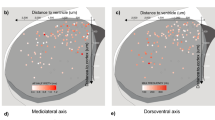
Dopaminergic neurons of the substantia nigra pars compacta (SNc) exhibit functional heterogeneity that likely underpins their diverse roles in behavior. We examined how the functional diversity of identified dopaminergic neurons in vivo correlates with differences in somato-dendritic architecture and afferent synaptic organization. Stereological analysis of individually recorded and labeled dopaminergic neurons of rat SNc revealed that they received approximately 8,000 synaptic inputs, at least 30% of which were glutamatergic and 40–70% were GABAergic. The latter synapses were proportionally greater in number and denser on dendrites located in the substantia nigra pars reticulata (SNr) than on those located in SNc, revealing the existence of two synaptically distinct and region-specific subcellular domains. We also found that the relative extension of SNc neuron dendrites into the SNr dictated overall GABAergic innervation and predicted inhibition responses to aversive stimuli. We conclude that diverse wiring patterns determine the heterogeneous activities of midbrain dopaminergic neurons in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
05 March 2012
In the version of this article initially published online, the large arrowheads were undefined in Figure 3a; the Figure 3b legend stated that the proportion of GABAergic synapses made with dendrites located in the SNr was greater than that of GABAergic and glutamatergic synapses in the SNc, whereas it should have stated that it was greater than that of GABAergic synapses in SNc and glutamatergic synapses in the SNr; the Table 2 legend listed the number of neurons as five, contradicting the correct values given in the table itself; a spurious reference to a Figure 5i should have read Figure 5f; and the units on the y axis in Figure 5e were given as mm × 103 instead of mm. The errors have been corrected for the print, PDF and HTML versions of this article.
References
Wise, R.A. Roles for nigrostriatal–not just mesocorticolimbic–dopamine in reward and addiction. Trends Neurosci. 32, 517–524 (2009).
Schultz, W. Behavioral dopamine signals. Trends Neurosci. 30, 203–210 (2007).
Tsai, H.C. et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009).
Blythe, S.N., Wokosin, D., Atherton, J.F. & Bevan, M.D. Cellular mechanisms underlying burst firing in substantia nigra dopamine neurons. J. Neurosci. 29, 15531–15541 (2009).
Kitai, S.T., Shepard, P.D., Callaway, J.C. & Scroggs, R. Afferent modulation of dopamine neuron firing patterns. Curr. Opin. Neurobiol. 9, 690–697 (1999).
Brischoux, F., Chakraborty, S., Brierley, D.I. & Ungless, M.A. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. USA 106, 4894–4899 (2009).
Brown, M.T., Henny, P., Bolam, J.P. & Magill, P.J. Activity of neurochemically heterogeneous dopaminergic neurons in the substantia nigra during spontaneous and driven changes in brain state. J. Neurosci. 29, 2915–2925 (2009).
Bromberg-Martin, E.S., Matsumoto, M. & Hikosaka, O. Dopamine in motivational control: rewarding, aversive and alerting. Neuron 68, 815–834 (2010).
Matsumoto, M. & Hikosaka, O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459, 837–841 (2009).
Joshua, M., Adler, A., Mitelman, R., Vaadia, E. & Bergman, H. Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J. Neurosci. 28, 11673–11684 (2008).
Tepper, J.M. & Lee, C.R. GABAergic control of substantia nigra dopaminergic neurons. Prog. Brain Res. 160, 189–208 (2007).
Bolam, J.P., Francis, C.M. & Henderson, Z. Cholinergic input to dopaminergic neurons in the substantia nigra: a double immunocytochemical study. Neuroscience 41, 483–494 (1991).
Bolam, J.P. & Smith, Y. The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Res. 529, 57–78 (1990).
Lavoie, B. & Parent, A. Pedunculopontine nucleus in the squirrel monkey: cholinergic and glutamatergic projections to the substantia nigra. J. Comp. Neurol. 344, 232–241 (1994).
Smith, Y., Charara, A. & Parent, A. Synaptic innervation of midbrain dopaminergic neurons by glutamate-enriched terminals in the squirrel monkey. J. Comp. Neurol. 364, 231–253 (1996).
Smith, Y., Bevan, M.D., Shink, E. & Bolam, J.P. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience 86, 353–387 (1998).
Pinault, D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or meurobiotin. J. Neurosci. Methods 65, 113–136 (1996).
Ascoli, G.A. Mobilizing the base of neuroscience data: the case of neuronal morphologies. Nat. Rev. Neurosci. 7, 318–324 (2006).
Preston, R.J., McCrea, R.A., Chang, H.T. & Kitai, S.T. Anatomy and physiology of substantia nigra and retrorubral neurons studied by extra- and intracellular recording and by horseradish peroxidase labeling. Neuroscience 6, 331–344 (1981).
Fallon, J.H. & Loughlin, S.E. Substantia nigra. in The Rat Nervous System (ed. G. Paxinos) 215–238 (Academic Press, London, 1995).
Gerfen, C.R., Baimbridge, K.G. & Thibault, J. The neostriatal mosaic. III. Biochemical and developmental dissociation of patch-matrix mesostriatal systems. J. Neurosci. 7, 3935–3944 (1987).
West, M.J. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 22, 51–61 (1999).
Gundersen, H.J. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J. Microsc. 143, 3–45 (1986).
Naito, A. & Kita, H. The cortico-nigral projection in the rat: an anterograde tracing study with biotinylated dextran amine. Brain Res. 637, 317–322 (1994).
Omelchenko, N. & Sesack, S.R. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience 146, 1259–1274 (2007).
Corvaja, N., Doucet, G. & Bolam, J.P. Ultrastructure and synaptic targets of the raphe-nigral projection in the rat. Neuroscience 55, 417–427 (1993).
Smith, Y., Hazrati, L.N. & Parent, A. Efferent projections of the subthalamic nucleus in the squirrel monkey as studied by the PHA-L anterograde tracing method. J. Comp. Neurol. 294, 306–323 (1990).
Mann, E.O. & Paulsen, O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 30, 343–349 (2007).
Fiala, J.C., Spacek, J. & Harris, K.M. Dendrite structure. in Dendrites (eds. G.J. Stuart, N. Spruston & M. Hausser) 1–41 (Oxford University Press, Oxford, 2008).
Magee, J.C. Dendritic integration of excitatory synaptic input. Nat. Rev. Neurosci. 1, 181–190 (2000).
Comoli, E. et al. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat. Neurosci. 6, 974–980 (2003).
Borgius, L., Restrepo, C.E., Leao, R.N., Saleh, N. & Kiehn, O. A transgenic mouse line for molecular genetic analysis of excitatory glutamatergic neurons. Mol. Cell. Neurosci. 45, 245–257 (2010).
Wang, H.L. & Morales, M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur. J. Neurosci. 29, 340–358 (2009).
Iribe, Y., Moore, K., Pang, K.C. & Tepper, J.M. Subthalamic stimulation–induced synaptic responses in substantia nigra pars compacta dopaminergic neurons in vitro. J. Neurophysiol. 82, 925–933 (1999).
Hajós, M. & Greenfield, S.A. Synaptic connections between pars compacta and pars reticulata neurones: electrophysiological evidence for functional modules in the substantia nigra. Brain Res. 660, 216–224 (1994).
Spruston, N. Pyramidal neurons: dendritic structure and synaptic integration. Nat. Rev. Neurosci. 9, 206–221 (2008).
Megías, M., Emri, Z., Freund, T.F. & Gulyas, A.I. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102, 527–540 (2001).
Grace, A.A. & Bunney, B.S. Paradoxical GABA excitation of nigral dopaminergic cells: indirect mediation through reticulata inhibitory neurons. Eur. J. Pharmacol. 59, 211–218 (1979).
Gulácsi, A. et al. Cell type–specific differences in chloride-regulatory mechanisms and GABA(A) receptor–mediated inhibition in rat substantia nigra. J. Neurosci. 23, 8237–8246 (2003).
Paladini, C.A., Celada, P. & Tepper, J.M. Striatal, pallidal and pars reticulata evoked inhibition of nigrostriatal dopaminergic neurons is mediated by GABA(A) receptors in vivo. Neuroscience 89, 799–812 (1999).
Jhou, T.C., Fields, H.L., Baxter, M.G., Saper, C.B. & Holland, P.C. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61, 786–800 (2009).
Mailly, P., Charpier, S., Menetrey, A. & Deniau, J.M. Three-dimensional organization of the recurrent axon collateral network of the substantia nigra pars reticulata neurons in the rat. J. Neurosci. 23, 5247–5257 (2003).
Maeda, H. & Mogenson, G.J. Effects of peripheral stimulation on the activity of neurons in the ventral tegmental area, substantia nigra and midbrain reticular formation of rats. Brain Res. Bull. 8, 7–14 (1982).
Coizet, V., Dommett, E.J., Klop, E.M., Redgrave, P. & Overton, P.G. The parabrachial nucleus is a critical link in the transmission of short latency nociceptive information to midbrain dopaminergic neurons. Neuroscience 168, 263–272 (2010).
Lodge, D.J. The medial prefrontal and orbitofrontal cortices differentially regulate dopamine system function. Neuropsychopharmacology. 36, 1227–1236 (2011).
Bayer, V.E. & Pickel, V.M. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase immunoreactivity in rat ventral tegmental area. Brain Res. 559, 44–55 (1991).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates (Elselvier, Amsterdam, 2007).
Bolam, J.P. Experimental Neuroanatomy: a Practical Approach (IRL Press, Oxford, 1992).
Sadek, A.R., Magill, P.J. & Bolam, J.P. A single-cell analysis of intrinsic connectivity in the rat globus pallidus. J. Neurosci. 27, 6352–6362 (2007).
Acknowledgements
We are grateful to M. West for providing critical comments during the development of the single-cell stereological protocol. We also thank E. Norman, C. Francis, K. Whitworth, B. Micklem, S. Fernández, J. Mpodozis and G. Marín for expert technical assistance, and C. Morales and M. Quispe for statistical analysis. This work was supported by the Medical Research Council (UK), Parkinson's UK (grant G-0601, to J.P.B., P.J.M. and M.A.U.), and the Comisión Nacional de Investigación Científica y Tecnológica (Fondecyt grant 11100433, Conicyt, Chile to P.H.).
Author information
Authors and Affiliations
Contributions
P.H. performed the juxtacellular labeling of some neurons, carried out most of the immunohistochemical procedures, electron microscopic analysis, neuronal reconstructions and stereological analysis, analyzed most of the anatomical and some of the physiological data, prepared the figures, and wrote the manuscript. M.T.C.B. recorded and juxtacellularly labeled most of the neurons that we used, and performed most of the physiological analysis. A.N. carried out neuronal reconstructions in half of the neurons. M.F. performed the light microscopic stereological analysis of immunolabeled varicosities. M.A.U. provided important feedback during the development of the project and gave critical comments during the writing of the manuscript. P.J.M. supervised and contributed to the recording and juxtacellular labeling of neurons and the project in general, and provided insightful comments during the writing of the manuscript. J.P.B. supervised the entire project, provided expertise in immunohistochemistry and tissue processing, helped with ultrastructural analysis, and provided critical and insightful comments during the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Tables 1 and 2 (PDF 1231 kb)
Rights and permissions
About this article
Cite this article
Henny, P., Brown, M., Northrop, A. et al. Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nat Neurosci 15, 613–619 (2012). https://doi.org/10.1038/nn.3048
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3048
This article is cited by
-
Activation of RMTg projections to the VTA reverse cocaine-induced molecular adaptation in the reward system
Translational Psychiatry (2024)
-
Blockade of the Dopamine D3 Receptor Attenuates Opioids-Induced Addictive Behaviours Associated with Inhibiting the Mesolimbic Dopamine System
Neuroscience Bulletin (2023)
-
Susceptibility to chronic immobilization stress‐induced depressive-like behaviour in middle‐aged female mice and accompanying changes in dopamine D1 and GABAA receptors in related brain regions
Behavioral and Brain Functions (2021)
-
Drug addiction: from bench to bedside
Translational Psychiatry (2021)
-
Pain modulates dopamine neurons via a spinal–parabrachial–mesencephalic circuit
Nature Neuroscience (2021)