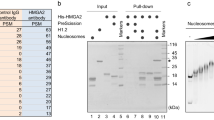
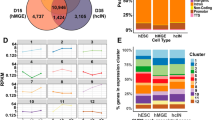
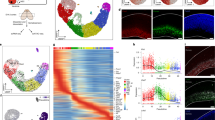
Abstract
Neural precursor cells (NPCs) in the mouse neocortex generate various neuronal and glial cell types in a developmental stage–dependent manner. Most NPCs lose their neurogenic potential during development, although the underlying mechanisms of this process are not fully understood. We found that the chromatin of mouse NPCs gradually becomes more condensed and less dynamic on a global scale during neocortical development. Furthermore, we found high mobility group A (HMGA) proteins to be essential for the open chromatin state of NPCs at early developmental stages. Knockdown of HMGA proteins in early-stage NPCs reduced their neurogenic potential. Conversely, overexpression of HMGA proteins conferred neurogenic potential on late-stage NPCs, an effect that was antagonized by coexpression of a histone H1 mutant that inhibits chromatin opening. Thus, HMGA proteins contribute to the neurogenic potential of NPCs in the early stages of neocortical development, possibly through induction of an open chromatin state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Meshorer, E. et al. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell 10, 105–116 (2006).
Gaspar-Maia, A. et al. Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature 460, 863–868 (2009).
Hajkova, P. et al. Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature 452, 877–881 (2008).
Seki, Y. et al. Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Dev. Biol. 278, 440–458 (2005).
Hirabayashi, Y. & Gotoh, Y. Stage-dependent fate determination of neural precursor cells in mouse forebrain. Neurosci. Res. 51, 331–336 (2005).
Miller, F.D. & Gauthier, A.S. Timing is everything: making neurons versus glia in the developing cortex. Neuron 54, 357–369 (2007).
Nagao, M. et al. Coordinated control of self-renewal and differentiation of neural stem cells by Myc and the p19ARF-p53 pathway. J. Cell Biol. 183, 1243–1257 (2008).
Hirabayashi, Y. et al. Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron 63, 600–613 (2009).
Naka, H., Nakamura, S., Shimazaki, T. & Okano, H. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat. Neurosci. 11, 1014–1023 (2008).
Sanosaka, T. et al. Identification of genes that restrict astrocyte differentiation of midgestational neural precursor cells. Neuroscience 155, 780–788 (2008).
Goldberg, A.D., Allis, C.D. & Bernstein, E. Epigenetics: a landscape takes shape. Cell 128, 635–638 (2007).
Fusco, A. & Fedele, M. Roles of HMGA proteins in cancer. Nat. Rev. Cancer 7, 899–910 (2007).
Reeves, R. Molecular biology of HMGA proteins: hubs of nuclear function. Gene 277, 63–81 (2001).
Qian, X. et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 28, 69–80 (2000).
Sunabori, T. et al. Cell cycle–specific nestin expression coordinates with morphological changes in embryonic cortical neural progenitors. J. Cell Sci. 121, 1204–1212 (2008).
Waterston, R.H. et al. Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562 (2002).
Catez, F. et al. Network of dynamic interactions between histone H1 and high-mobility-group proteins in chromatin. Mol. Cell. Biol. 24, 4321–4328 (2004).
Zhao, K., Käs, E., Gonzalez, E. & Laemmli, U.K. SAR-dependent mobilization of histone H1 by HMG-I/Y in vitro: HMG-I/Y is enriched in H1-depleted chromatin. EMBO J. 12, 3237–3247 (1993).
Nishino, J., Kim, I., Chada, K. & Morrison, S.J. Hmga2 promotes neural stem cell self-renewal in young, but not old, mice by reducing p16Ink4a and p19Arf expression. Cell 135, 227–239 (2008).
Zhou, X., Benson, K.F., Ashar, H.R. & Chada, K. Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature 376, 771–774 (1995).
Gloster, A. et al. The T alpha 1 alpha-tubulin promoter specifies gene expression as a function of neuronal growth and regeneration in transgenic mice. J. Neurosci. 14, 7319–7330 (1994).
Sawamoto, K. et al. Direct isolation of committed neuronal progenitor cells from transgenic mice coexpressing spectrally distinct fluorescent proteins regulated by stage-specific neural promoters. J. Neurosci. Res. 65, 220–227 (2001).
Ramirez-Carrozzi, V.R. et al. A unifying model for the selective regulation of inducible transcription by CpG islands and nucleosome remodeling. Cell 138, 114–128 (2009).
Hirabayashi, Y. & Gotoh, Y. Epigenetic control of neural precursor cell fate during development. Nat. Rev. Neurosci. 11, 377–388 (2010).
Frisch, M. et al. In silico prediction of scaffold/matrix attachment regions in large genomic sequences. Genome Res. 12, 349–354 (2002).
Knoepfler, P.S. et al. Myc influences global chromatin structure. EMBO J. 25, 2723–2734 (2006).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Chang, T.C. et al. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc. Natl. Acad. Sci. USA 106, 3384–3389 (2009).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Heo, I. et al. Lin28 mediates the terminal uridylation of let-7 precursor microRNA. Mol. Cell 32, 276–284 (2008).
Newman, M.A., Thomson, J.M. & Hammond, S.M. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA 14, 1539–1549 (2008).
Piskounova, E. et al. Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J. Biol. Chem. 283, 21310–21314 (2008).
Rybak, A. et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat. Cell Biol. 10, 987–993 (2008).
Viswanathan, S.R., Daley, G.Q. & Gregory, R.I. Selective blockade of microRNA processing by Lin28. Science 320, 97–100 (2008).
Lee, Y.S. & Dutta, A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 21, 1025–1030 (2007).
Mayr, C., Hemann, M.T. & Bartel, D.P. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 315, 1576–1579 (2007).
Hirabayashi, Y. et al. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development 131, 2791–2801 (2004).
Konno, D. et al. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat. Cell Biol. 10, 93–101 (2008).
Miyata, T. & Ogawa, M. Twisting of neocortical progenitor cells underlies a spring-like mechanism for daughter-cell migration. Curr. Biol. 17, 146–151 (2007).
Ochiai, W. et al. Periventricular notch activation and asymmetric Ngn2 and Tbr2 expression in pair-generated neocortical daughter cells. Mol. Cell. Neurosci. 40, 225–233 (2009).
Tabata, H. & Nakajima, K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience 103, 865–872 (2001).
Kamakura, S. et al. Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signaling. Nat. Cell Biol. 6, 547–554 (2004).
Watakabe, A. et al. Comparative analysis of layer-specific genes in Mammalian neocortex. Cereb. Cortex 17, 1918–1933 (2007).
Kuwahara, A. et al. Wnt signaling and its downstream target N-myc regulate basal progenitors in the developing neocortex. Development 137, 1035–1044 (2010).
Acknowledgements
We thank C.L. Cepko (Harvard University), T. Matsuda (Kyoto University), T. Kitamura (The University of Tokyo), M. Nakafuku (Cincinnati Children's Hospital Medical Center and University of Cincinnati), H. Song (Johns Hopkins University) and H. Okano (Keio University) for plasmids, K. Ohsumi (Nagoya University) for antibodies, K. Tyssowski and E. Nigh for editing the manuscript, H. Okano for Nes-d4Venus mice, A. Miyajima for FACS, and members of the Gotoh laboratory for helpful discussion. This work was supported by Grants-in-Aid for Scientific Research (A) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan, Innovative Areas “Neural Diversity and Neocortical Organization” of MEXT, by Core Research for Evolutional Science and Technology of the Japan Science and Technology Agency, by the Japan Society for the Promotion of Science, and by the Global COE Program “Integrative Life Science Based on the Study of Biosignaling Mechanisms” of MEXT.
Author information
Authors and Affiliations
Contributions
Y.K., Y.F., Y.H. and Y.G. designed the study. Y.K. and Y.F. carried out the experiments and analyzed the data. Y.H. supported the experiments. Y.K. and Y.G. wrote the manuscript. Y.G. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 6220 kb)
Rights and permissions
About this article
Cite this article
Kishi, Y., Fujii, Y., Hirabayashi, Y. et al. HMGA regulates the global chromatin state and neurogenic potential in neocortical precursor cells. Nat Neurosci 15, 1127–1133 (2012). https://doi.org/10.1038/nn.3165
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3165
This article is cited by
-
An epigenetic barrier sets the timing of human neuronal maturation
Nature (2024)
-
HMGA2 directly mediates chromatin condensation in association with neuronal fate regulation
Nature Communications (2023)
-
SUPPRESSOR OF PHYTOCHROME B-4 #3 reduces the expression of PIF-activated genes and increases expression of growth repressors to regulate hypocotyl elongation in short days
BMC Plant Biology (2022)
-
Epithelial cells-enriched lncRNA SNHG8 regulates chromatin condensation by binding to Histone H1s
Cell Death & Differentiation (2022)
-
An Arabidopsis AT-hook motif nuclear protein mediates somatic embryogenesis and coinciding genome duplication
Nature Communications (2021)