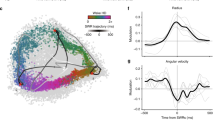
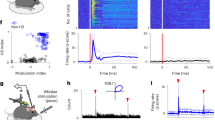
Abstract
High-level cortical systems for spatial navigation, including entorhinal grid cells, critically depend on input from the head direction system. We examined spiking rhythms and modes of synchrony between neurons participating in head direction networks for evidence of internal processing, independent of direct sensory drive, which may be important for grid cell function. We found that head direction networks of rats were segregated into at least two populations of neurons firing on alternate theta cycles (theta cycle skipping) with fixed synchronous or anti-synchronous relationships. Pairs of anti-synchronous theta cycle skipping neurons exhibited larger differences in head direction tuning, with a minimum difference of 40 degrees of head direction. Septal inactivation preserved the head direction signal, but eliminated theta cycle skipping of head direction cells and grid cell spatial periodicity. We propose that internal mechanisms underlying cycle skipping in head direction networks may be critical for downstream spatial computation by grid cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Buzsáki, G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron 68, 362–385 (2010).
Green, J.D. & Arduini, A.A. Hippocampal electrical activity in arousal. J. Neurophysiol. 17, 533–557 (1954).
Vertes, R.P. & Kocsis, B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience 81, 893–926 (1997).
Hasselmo, M.E. What is the function of hippocampal theta rhythm? Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus 15, 936–949 (2005).
Winson, J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 201, 160–163 (1978).
Kahana, M.J. et al. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399, 781–784 (1999).
Givens, B. & Olton, D.S. Bidirectional modulation of scopolamine-induced working memory impairments by muscarinic activation of the medial septal area. Neurobiol. Learn. Mem. 63, 269–276 (1995).
Seager, M.A. et al. Oscillatory brain states and learning: Impact of hippocampal theta-contingent training. Proc. Natl. Acad. Sci. USA 99, 1616–1620 (2002).
Givens, B.S. & Olton, D.S. Cholinergic and GABAergic modulation of the medial septal area: effect on working memory. Behav. Neurosci. 104, 849–855 (1990).
Chrobak, J.J., Stackman, R.W. & Walsh, T.J. Intraseptal administration of muscimol produces dose-dependent memory impairments in the rat. Behav. Neural Biol. 52, 357–369 (1989).
Klausberger, T. et al. Brain state– and cell type–specific firing of hippocampal interneurons in vivo. Nature 421, 844–848 (2003).
Mizuseki, K. et al. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron 64, 267–280 (2009).
O'Keefe, J. & Recce, M.L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330 (1993).
Skaggs, W.E. et al. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172 (1996).
Hafting, T. et al. Hippocampus-independent phase precession in entorhinal grid cells. Nature 453, 1248–1252 (2008).
O'Keefe, J. & Burgess, N. Dual phase and rate coding in hippocampal place cells: theoretical significance and relationship to entorhinal grid cells. Hippocampus 15, 853–866 (2005).
Burgess, N., Barry, C. & O'Keefe, J. An oscillatory interference model of grid cell firing. Hippocampus 17, 801–812 (2007).
Hasselmo, M.E., Giocomo, L.M. & Zilli, E.A. Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons. Hippocampus 17, 1252–1271 (2007).
Mehta, M.R., Lee, A.K. & Wilson, M.A. Role of experience and oscillations in transforming a rate code into a temporal code. Nature 417, 741–746 (2002).
Jensen, O. & Lisman, J.E. Novel lists of 7 ± 2 known items can be reliably stored in an oscillatory short-term memory network: interaction with long-term memory. Learn. Mem. 3, 257–263 (1996).
Wallenstein, G.V. & Hasselmo, M.E. GABAergic modulation of hippocampal population activity: sequence learning, place field development, and the phase precession effect. J. Neurophysiol. 78, 393–408 (1997).
Tsodyks, M.V. et al. Population dynamics and theta rhythm phase precession of hippocampal place cell firing: a spiking neuron model. Hippocampus 6, 271–280 (1996).
Burgess, N. Grid cells and theta as oscillatory interference: theory and predictions. Hippocampus 18, 1157–1174 (2008).
Welday, A.C. et al. Cosine directional tuning of theta cell burst frequencies: evidence for spatial coding by oscillatory interference. J. Neurosci. 31, 16157–16176 (2011).
McNaughton, B.L. et al. Path integration and the neural basis of the 'cognitive map'. Nat. Rev. Neurosci. 7, 663–678 (2006).
Navratilova, Z. et al. Phase precession and variable spatial scaling in a periodic attractor map model of medial entorhinal grid cells with realistic after-spike dynamics. Hippocampus 22, 772–789 (2012).
Taube, J.S. Head direction cells and the neurophysiological basis for a sense of direction. Prog. Neurobiol. 55, 225–256 (1998).
Taube, J.S. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J. Neurosci. 15, 70–86 (1995).
Taube, J.S., Muller, R.U. & Ranck, J.B. Jr. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 10, 420–435 (1990).
Cho, J. & Sharp, P.E. Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behav. Neurosci. 115, 3–25 (2001).
Boccara, C.N. et al. Grid cells in pre- and parasubiculum. Nat. Neurosci. 13, 987–994 (2010).
Giocomo, L.M. et al. Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing. Science 315, 1719–1722 (2007).
Sargolini, F. et al. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312, 758–762 (2006).
Burgalossi, A. et al. Microcircuits of functionally identified neurons in the rat medial entorhinal cortex. Neuron 70, 773–786 (2011).
Jeffery, K.J., Donnett, J.G. & O'Keefe, J. Medial septal control of theta-correlated unit firing in the entorhinal cortex of awake rats. Neuroreport 6, 2166–2170 (1995).
Deshmukh, S.S. et al. Theta modulation in the medial and the lateral entorhinal cortices. J. Neurophysiol. 104, 994–1006 (2010).
Fujisawa, S. & Buzsaki, G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA and hippocampal activities. Neuron 72, 153–165 (2011).
Gevins, A. et al. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing and practice. Cereb. Cortex 7, 374–385 (1997).
Brandon, M.P. et al. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science 332, 595–599 (2011).
Harris, K.D. et al. Organization of cell assemblies in the hippocampus. Nature 424, 552–556 (2003).
Royer, S. et al. Distinct representations and theta dynamics in dorsal and ventral hippocampus. J. Neurosci. 30, 1777–1787 (2010).
Brandon, M.P. et al. Head direction cells in the postsubiculum do not show replay of prior waking sequences during sleep. Hippocampus 22, 604–618 (2012).
Harris, K.D. et al. Spike train dynamics predicts theta-related phase precession in hippocampal pyramidal cells. Nature 417, 738–741 (2002).
Huxter, J., Burgess, N. & O'Keefe, J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature 425, 828–832 (2003).
King, C., Reece, M. & O'Keefe, J. The rhythmicity of cells of the medial septum/diagonal band of Broca in the awake freely moving rat: relationships with behaviour and hippocampal theta. Eur. J. Neurosci. 10, 464–477 (1998).
Hasselmo, M.E. & Brandon, M.P. A model combining oscillations and attractor dynamics for generation of grid cell firing. Front. Neural Circuits 6, 30 (2012).
Jezek, K. et al. Theta-paced flickering between place-cell maps in the hippocampus. Nature 478, 246–249 (2011).
Colgin, L.L. et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462, 353–357 (2009).
Lisman, J. & Buzsaki, G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr. Bull. 34, 974–980 (2008).
Howard, M.W. et al. Gamma oscillations correlate with working memory load in humans. Cereb. Cortex 13, 1369–1374 (2003).
Taube, J.S., Muller, R.U. & Ranck, J.B. Jr. Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J. Neurosci. 10, 436–447 (1990).
Acknowledgements
We kindly thank S. Gillet, J. Hinman, E. Newman and L. Ewell for their invaluable consultations and comments on previous versions of this manuscript, as well as M. Connerney, S. Eriksson, C. Libby and T. Ware for technical assistance and behavioral training. This work was supported by grants from the National Institute of Mental Health (R01 MH60013 and MH61492) and the Office of Naval Research Multidisciplinary University Research Initiative (N00014-10-1-0936).
Author information
Authors and Affiliations
Contributions
M.P.B. and M.E.H. designed the in vivo experiments. M.P.B. collected the in vivo data. M.P.B., A.R.B. and N.W.S. designed, and A.R.B. implemented, the in vivo analyses. N.W.S. and M.E.H. designed the in vitro experiments. N.W.S. collected and analyzed the in vitro data. M.E.H. created the network simulations and A.R.B. developed the Poisson model. M.P.B., A.R.B., N.W.S. and M.E.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Modeling (PDF 1620 kb)
Rights and permissions
About this article
Cite this article
Brandon, M., Bogaard, A., Schultheiss, N. et al. Segregation of cortical head direction cell assemblies on alternating theta cycles. Nat Neurosci 16, 739–748 (2013). https://doi.org/10.1038/nn.3383
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3383
This article is cited by
-
Enhanced hippocampal theta rhythmicity and emergence of eta oscillation in virtual reality
Nature Neuroscience (2021)
-
Navigating for reward
Nature Reviews Neuroscience (2021)
-
Rhythms of the hippocampal network
Nature Reviews Neuroscience (2016)
-
Three-dimensional head-direction coding in the bat brain
Nature (2015)