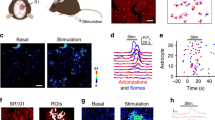
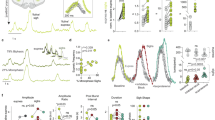
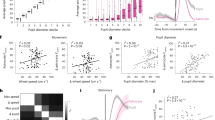
Abstract
Communication between neurons rests on their capacity to change their firing pattern to encode different messages. For several vital functions, such as respiration and mastication, neurons need to generate a rhythmic firing pattern. Here we show in the rat trigeminal sensori-motor circuit for mastication that this ability depends on regulation of the extracellular Ca2+ concentration ([Ca2+]e) by astrocytes. In this circuit, astrocytes respond to sensory stimuli that induce neuronal rhythmic activity, and their blockade with a Ca2+ chelator prevents neurons from generating a rhythmic bursting pattern. This ability is restored by adding S100β, an astrocytic Ca2+-binding protein, to the extracellular space, while application of an anti-S100β antibody prevents generation of rhythmic activity. These results indicate that astrocytes regulate a fundamental neuronal property: the capacity to change firing pattern. These findings may have broad implications for many other neural networks whose functions depend on the generation of rhythmic activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Grillner, S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiol. Rev. 55, 247–304 (1975).
Dellow, P.G. & Lund, J.P. Evidence for central timing of rhythmical mastication. J. Physiol. (Lond.) 215, 1–13 (1971).
Smith, J.C., Ellenberger, H.H., Ballanyi, K., Richter, D.W. & Feldman, J.L. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729 (1991).
Harris-Warrick, R.M. General principles of rhythmogenesis in central pattern generator networks. Prog. Brain Res. 187, 213–222 (2010).
Del Negro, C.A. et al. Synaptically activated burst-generating conductances may underlie a group-pacemaker mechanism for respiratory rhythm generation in mammals. Prog. Brain Res. 187, 111–136 (2010).
Gourine, A.V. et al. Astrocytes control breathing through pH-dependent release of ATP. Science 329, 571–575 (2010).
Okada, Y. et al. Preinspiratory calcium rise in putative pre-Botzinger complex astrocytes. J. Physiol. (Lond.) 590, 4933–4944 (2012).
Tazerart, S., Vinay, L. & Brocard, F. The persistent sodium current generates pacemaker activities in the central pattern generator for locomotion and regulates the locomotor rhythm. J. Neurosci. 28, 8577–8589 (2008).
Brocard, F., Verdier, D., Arsenault, I., Lund, J.P. & Kolta, A. Emergence of intrinsic bursting in trigeminal sensory neurons parallels the acquisition of mastication in weanling rats. J. Neurophysiol. 96, 2410–2424 (2006).
Massimini, M. & Amzica, F. Extracellular calcium fluctuations and intracellular potentials in the cortex during the slow sleep oscillation. J. Neurophysiol. 85, 1346–1350 (2001).
Brocard, F. et al. Activity-dependent changes in extracellular Ca2+ and K+ reveal pacemakers in the spinal locomotor-related network. Neuron 77, 1047–1054 (2013).
Trulsson, M. & Johansson, R.S. Orofacial mechanoreceptors in humans: encoding characteristics and responses during natural orofacial behaviors. Behav. Brain Res. 135, 27–33 (2002).
Westneat, M.W. & Hall, W.G. Ontogeny of feeding motor patterns in infant rats– an electromyographic analysis of suckling and chewing. Behav. Neurosci. 106, 539–554 (1992).
Bernier, A.P., Arsenault, I., Lund, J.P. & Kolta, A. Effect of the stimulation of sensory inputs on the firing of neurons of the trigeminal main sensory nucleus in the rat. J. Neurophysiol. 103, 915–923 (2010).
Crill, W.E. Persistent sodium current in mammalian central neurons. Annu. Rev. Physiol. 58, 349–362 (1996).
Jones, H.C. & Keep, R.F. Brain fluid calcium concentration and response to acute hypercalcaemia during development in the rat. J. Physiol. (Lond.) 402, 579–593 (1988).
Li, Z. & Hatton, G.I. Oscillatory bursting of phasically firing rat supraoptic neurones in low-Ca2+ medium: Na+ influx, cytosolic Ca2+ and gap junctions. J. Physiol. (Lond.) 496, 379–394 (1996).
Su, H., Alroy, G., Kirson, E.D. & Yaari, Y. Extracellular calcium modulates persistent sodium current-dependent burst-firing in hippocampal pyramidal neurons. J. Neurosci. 21, 4173–4182 (2001).
Torres, A. et al. Extracellular Ca2+ acts as a mediator of communication from neurons to glia. Sci. Signal. 5, ra8 (2012).
Wang, F. et al. Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci. Signal. 5, ra26 (2012).
Lazarov, N.E. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog. Neurobiol. 66, 19–59 (2002).
Serrano, A., Haddjeri, N., Lacaille, J.C. & Robitaille, R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J. Neurosci. 26, 5370–5382 (2006).
Ciccarelli, R. et al. Activation of A1 adenosine or mGlu3 metabotropic glutamate receptors enhances the release of nerve growth factor and S-100beta protein from cultured astrocytes. Glia 27, 275–281 (1999).
Sakatani, S. et al. Neural-activity-dependent release of S100B from astrocytes enhances kainate-induced gamma oscillations in vivo. J. Neurosci. 28, 10928–10936 (2008).
Markowitz, J. et al. Calcium-binding properties of wild-type and EF-hand mutants of S100B in the presence and absence of a peptide derived from the C-terminal negative regulatory domain of p53. Biochemistry 44, 7305–7314 (2005).
Egelman, D.M. & Montague, P.R. Calcium dynamics in the extracellular space of mammalian neural tissue. Biophys. J. 76, 1856–1867 (1999).
Lian, X.Y. & Stringer, J.L. Astrocytes contribute to regulation of extracellular calcium and potassium in the rat cerebral cortex during spreading depression. Brain Res. 1012, 177–184 (2004).
Nicholson, C., Bruggencate, G.T., Steinberg, R. & Stockle, H. Calcium modulation in brain extracellular microenvironment demonstrated with ion-selective micropipette. Proc. Natl. Acad. Sci. USA 74, 1287–1290 (1977).
Jefferys, J.G. & Haas, H.L. Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature 300, 448–450 (1982).
Del Negro, C.A., Morgado-Valle, C. & Feldman, J.L. Respiratory rhythm: an emergent network property? Neuron 34, 821–830 (2002).
Armstrong, C.M. Distinguishing surface effects of calcium ion from pore-occupancy effects in Na+ channels. Proc. Natl. Acad. Sci. USA 96, 4158–4163 (1999).
Rybak, I.A., Shevtsova, N.A., St-John, W.M., Paton, J.F. & Pierrefiche, O. Endogenous rhythm generation in the pre-Botzinger complex and ionic currents: modelling and in vitro studies. Eur. J. Neurosci. 18, 239–257 (2003).
Tsuruyama, K., Hsiao, C.F. & Chandler, S.H. Participation of a persistent sodium current and calcium-activated nonspecific cationic current to burst generation in trigeminal principal sensory neurons. J. Neurophysiol. 110, 1903–1914 (2013).
Panatier, A. et al. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798 (2011).
Pasti, L., Volterra, A., Pozzan, T. & Carmignoto, G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J. Neurosci. 17, 7817–7830 (1997).
Lee, M.C. et al. Characterisation of the expression of NMDA receptors in human astrocytes. PLoS ONE 5, e14123 (2010).
Sigvardt, K.A., Grillner, S., Wallen, P. & Van Dongen, P.A. Activation of NMDA receptors elicits fictive locomotion and bistable membrane properties in the lamprey spinal cord. Brain Res. 336, 390–395 (1985).
Kim, Y.I. & Chandler, S.H. NMDA-induced burst discharge in guinea pig trigeminal motoneurons in vitro. J. Neurophysiol. 74, 334–346 (1995).
Donato, R. et al. S100B's double life: intracellular regulator and extracellular signal. Biochim. Biophys. Acta 1793, 1008–1022 (2009).
Capra, N.F. & Dessem, D. Central connections of trigeminal primary afferent neurons: topographical and functional considerations. Crit. Rev. Oral. Biol. Med. 4, 1–52 (1992).
Yamamura, K. et al. Effects of reversible bilateral inactivation of face primary motor cortex on mastication and swallowing. Brain Res. 944, 40–55 (2002).
Lund, J.P. & Dellow, P.G. The influence of interactive stimuli on rhythmical masticatory movements in rabbits. Arch. Oral Biol. 16, 215–223 (1971).
Di Prisco, G.V., Pearlstein, E., Robitaille, R. & Dubuc, R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science 278, 1122–1125 (1997).
Tsuboi, A., Kolta, A., Chen, C.C. & Lund, J.P. Neurons of the trigeminal main sensory nucleus participate in the generation of rhythmic motor patterns. Eur. J. Neurosci. 17, 229–238 (2003).
Kasymov, V. et al. Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astroglia. J. Neurosci. 33, 435–441 (2013).
Gomez-Gonzalo, M. et al. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 8, e1000352 (2010).
Pumain, R., Menini, C., Heinemann, U., Louvel, J. & Silva-Barrat, C. Chemical synaptic transmission is not necessary for epileptic seizures to persist in the baboon Papio papio. Exp. Neurol. 89, 250–258 (1985).
Kafitz, K.W., Meier, S.D., Stephan, J. & Rose, C.R. Developmental profile and properties of sulforhodamine 101–labeled glial cells in acute brain slices of rat hippocampus. J. Neurosci. Methods 169, 84–92 (2008).
Acknowledgements
This paper is dedicated to Laurent Vinay, whose premature loss leaves us with a tremendous void. To a great man who left his mark in this field by his science and his humanity. We are extremely grateful to D. Weber and P. Wilder from the Center for Biomolecular Therapeutics for generously providing the mutated S100β, which was well characterized in their previous work. We are equally grateful to J.G. Omichinski for giving us access to his Microcal ITC-200 microcalorimeter and for counseling us on these experiments. We also thank A. Panatier for counseling and assistance in several experiments on astrocytes and F. Amzica, who guided us for the ion-sensitive recordings. S. Condamine generously performed the immunostaining of S100β in NVsnpr. P.M. received a fellowship from the Network for Oral Health and Bone Health Research of the Fonds de Recherche Québec-Santé. This research was financed by a grant from the Canadian Institutes for Health Research (grant 14392).
Author information
Authors and Affiliations
Contributions
The work presented here was carried out in collaboration between all authors. All authors have contributed to, seen and approved the manuscript. A. Kolta and R.R. cosupervised the project and worked together to define the research themes, design the experiments and draft the manuscript (writing and critical revision). P.M. co-designed and carried out the patch recording and Ca2+ imaging experiments and worked on the data analysis, the interpretation of the results, and the drafting of the manuscript (writing and figures conception). D.V. co-designed and carried out part of the patch recording experiments, co-designed and carried out the interface configuration experiments, and worked on the data analysis, interpretation of the results and drafting of the manuscript (writing and figure concepts). A. Kadala co-designed and carried out the calcium ion–sensitive recording experiments and worked on the data analysis, the interpretation of the results and the drafting of the manuscript (writing and figure concepts). J.F. synthesized the S100β and conducted the microcalorimetry experiments and analyzed the related data. A.G.P. carried out some of the patch experiments and worked on the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Distinction among different firing patterns.
Examples of whole cell recordings (top) and extracellular recordings (bottom) showing the regular low-frequency firing referred to as tonic firing (left) and the rhythmic bursting firing (right). Bursts are clusters of at least 3 spikes occurring at high frequency separated by silent periods. In whole cell rhythmic records, these spikes appeared on top of depolarizing plateaus. Doublets of spikes (middle), where an ADP following the first spike supports a second spike, are often recorded in transition between these two types of firing patterns. ISI = interspike interval; IBI = interburst interval.
Supplementary Figure 2 Modulation of the pharmacologically isolated INaP by local applications of substances that reduce [Ca2+]e
(a) The inward current induced by voltage ramps from –80 to 0 mV (lower trace), after leak subtraction, under control conditions (black) is enhanced during local application of BAPTA (10 mM) (blue, n = 6 cells in 4 slices from 4 rats, control: 126.69 ± 40 pA vs BAPTA: 188 ± 54 pA, paired t-test; P = 0.021) and blocked by riluzole (20 µM) (grey, n = 4 cells in 4 slices from 3 rats). (b) The histograms illustrate the amplitude of the peak inward current during local application of BAPTA (n = 6 cells in 4 slices from 4 rats) and S100β (n = 7 cells in 5 slices from 5 rats) normalized to the control (n = 11 cells in 7 slices from 7 rats). (c) Pharmacologically-isolated trace of INaP obtained after subtracting the trace obtained with BAPTA and riluzole from the trace obtained with BAPTA alone (same cell as in (a)). (d) Voltage-dependency of INaP activation under control conditions (black) and during BAPTA (blue) and S100β (purple) application. INaP was normalized to the maximal value and fitted with a single Boltzmann function. Controls: V1/2max = –53.8 ± 0.4 mV, slope factor of the fitted curve k = 7.4. BAPTA: V1/2max = –60.2 ± 0.5 mV and k = 5.8. S100β: V1/2max = –57.8 ± 0.7 mV and k = 6.1. Conductance was calculated with G = I/(V – Erev). Erev = –65 mV, based on the concentration of extracellular and intracellular pipette solutions.
Supplementary Figure 3 Neuronal NVsnpr bursting does not depend on a purinergic mechanism.
A tonically firing NVsnpr neuron (left) displayed rhythmic bursting after local extracellular application of BAPTA (10 mM; right) in presence of bath-applied Suramine (50 µM, n = 5/5 cells in 3 slices from 1 rat), a purinergic receptors antagonist, indicating that NVsnpr neuronal bursting does not depend on a purinergic mechanism triggered by the [Ca2+]e decrease.
Supplementary Figure 4 The effects of diffusion of intracellular BAPTA in the astrocytic syncytium depend on the concentration of BAPTA used and are occluded in Ca2+-free aCSF.
(a) Dialysis of an astrocyte with a low concentration of BAPTA (0.1 mM) does not prevent bursting in the adjacent neuron, even after one hour (n = 4/4 pairs in 4 slices from 4 rats). (b) Spontaneous rhythmic bursting of an NVsnpr neuron recorded in Ca2+-free aCSF (top left) persisted after dialysis of an adjacent astrocyte (green trace) with BAPTA (20 mM, bottom left, n = 3 pairs in 3 slices from 3 rats) consistent with an astrocytic regulation of neuronal bursting by decreasing [Ca2+]e.
Supplementary Figure 5 Immunostaining of S100β in dorsal NVsnpr.
The dorsal part of NVsnpr (box in the image on left) contains a large number of immunoreactive astrocytes when an antibody against S100β is used.
Supplementary Figure 6 Binding of Ca2+ to S100β is prevented in presence of an antibody.
Isothermal titration calorimetry data for the titration of S100β with Ca2+ in the absence (a) or the presence (b) of an anti-S100β antibody. Top panel: raw thermogram of S100β titrated with CaCl2 at 20°C. Bottom panel: Integrated heats of the raw data from the top panel.
Supplementary Figure 7 Effects of S100β depend on its ability to reduce extracellular calcium.
(a) Local application of the Ca2+-free buffer used to dilute S100β induces a small decrease of [Ca2+]e (n = 6 recording sites in 6 slices from 3 rats), but does not cause bursting in NVsnpr neurons, in neither the interface ((b) extracellular recording; right trace, n = 11 cells in 11 slices from 6 rats) or the submerged configuration ((c) intracellular recording; right trace, n = 3 cells in 3 slices from 2 rats). (d) Local application of S100β diluted in the Ca2+-free buffer elicits neuronal bursting (left trace) in a cell from a preparation submerged in normal aCSF (with 1.6 mM Ca2+), but not after raising the [Ca2+]e in the aCSF to 2.6 mM (n = 4 cells in 4 slices from 3 rats). (e) Local application of S100β diluted in a buffer containing 1.6 mM Ca2+ can still elicit bursting, when its concentration is increased to1 mM (right trace (n = 10 cells in 4 slices from 4 rats) to prevent its saturation, but not at the concentration of 129 µM; left trace (n = 5 cells in 3 slices from 3 rats).
Supplementary Figure 8 Model of the sequence of events leading to rhythmogenesis.
Left: Low sensory activity level are insufficient to activate astrocytes and lower [Ca2+]e, thus preventing activation of INaP and neuronal bursting. In this condition, NVsnpr neurons work in a sensory relay mode with their tonic output faithfully relaying their tonic input. Right: With food intake and intraoral stimulation, sensory inputs from the periodontal ligament and the jaw muscle spindles increase their activity level and signal the need for rhythmic mastication. This increased activity activates astrocytes and leads to release of the Ca2+-binding protein S100β and subsequent decrease of [Ca2+]e. This in turn will activate INaP and elicit rhythmic bursting in NVsnpr neurons. The generated bursting frequency and pattern, reflecting the pattern of sensory inputs will then be transmitted to jaw closing and opening motoneuronal pools (masseter (mass) and digastric (dig), respectively).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 3077 kb)
Rights and permissions
About this article
Cite this article
Morquette, P., Verdier, D., Kadala, A. et al. An astrocyte-dependent mechanism for neuronal rhythmogenesis. Nat Neurosci 18, 844–854 (2015). https://doi.org/10.1038/nn.4013
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4013
This article is cited by
-
Synchronization of inspiratory burst onset along the ventral respiratory column in the neonate mouse is mediated by electrotonic coupling
BMC Biology (2023)
-
A conceptual framework for astrocyte function
Nature Neuroscience (2023)
-
Imaging the time course, morphology, neuronal tissue compression, and resolution of cerebral microhemorrhages in mice using intravital two-photon microscopy: insights into arteriolar, capillary, and venular origin
GeroScience (2023)
-
Diagnostic biomarker kinetics: how brain-derived biomarkers distribute through the human body, and how this affects their diagnostic significance: the case of S100B
Fluids and Barriers of the CNS (2022)
-
Astrocytes in Post-traumatic Stress Disorder
Neuroscience Bulletin (2022)