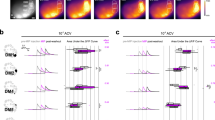
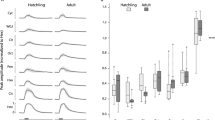
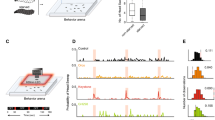
Abstract
In both the vertebrate nose and the insect antenna, most olfactory receptor neurons (ORNs) respond to multiple odors. However, some ORNs respond to just a single odor, or at most to a few highly related odors. It has been hypothesized that narrowly tuned ORNs project to narrowly tuned neurons in the brain, and that these dedicated circuits mediate innate behavioral responses to a particular ligand. Here we have investigated neural activity and behavior downstream from two narrowly tuned ORN types in Drosophila melanogaster. We found that genetically ablating either of these ORN types impairs innate behavioral attraction to their cognate ligand. Neurons in the antennal lobe postsynaptic to one of these ORN types are, like their presynaptic ORNs, narrowly tuned to a pheromone. However, neurons postsynaptic to the second ORN type are broadly tuned. These results demonstrate that some narrowly tuned ORNs project to dedicated central circuits, ensuring a tight connection between stimulus and behavior, whereas others project to central neurons that participate in the ensemble representations of many odors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Duchamp-Viret, P., Chaput, M.A. & Duchamp, A. Odor response properties of rat olfactory receptor neurons. Science 284, 2171–2174 (1999).
Malnic, B., Hirono, J., Sato, T. & Buck, L.B. Combinatorial receptor codes for odors. Cell 96, 713–723 (1999).
de Bruyne, M., Foster, K. & Carlson, J.R. Odor coding in the Drosophila antenna. Neuron 30, 537–552 (2001).
Hildebrand, J.G. & Shepherd, G.M. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu. Rev. Neurosci. 20, 595–631 (1997).
Wilson, R.I. & Mainen, Z.F. Early events in olfactory processing. Annu. Rev. Neurosci. 29, 163–201 (2006).
Boeckh, J., Kaissling, K.E. & Schneider, D. Insect olfactory receptors. Cold Spring Harb. Symp. Quant. Biol. 30, 263–280 (1965).
Schneider, D. & Steinbrecht, R.A Checklist of insect olfactory sensilla. Symp. Zool. Soc. Lond. 23, 279–297 (1968).
Suh, G.S. et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 431, 854–859 (2004).
Friedrich, R.W. & Korsching, S.I. Chemotopic, combinatorial and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. J. Neurosci. 18, 9977–9988 (1998).
Mombaerts, P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat. Rev. Neurosci. 5, 263–278 (2004).
Clyne, P., Grant, A., O'Connell, R. & Carlson, J.R. Odorant response of individual sensilla on the Drosophila antenna. Invert. Neurosci. 3, 127–135 (1997).
de Bruyne, M., Clyne, P.J. & Carlson, J.R. Odor coding in a model olfactory organ: the Drosophila maxillary palp. J. Neurosci. 19, 4520–4532 (1999).
Laissue, P.P. et al. Three-dimensional reconstruction of the antennal lobe in Drosophila melanogaster. J. Comp. Neurol. 405, 543–552 (1999).
Hallem, E.A., Ho, M.G. & Carlson, J.R. The molecular basis of odor coding in the Drosophila antenna. Cell 117, 965–979 (2004).
Couto, A., Alenius, M. & Dickson, B.J. Molecular, anatomical and functional organization of the Drosophila olfactory system. Curr. Biol. 15, 1535–1547 (2005).
Fishilevich, E. & Vosshall, L.B. Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 15, 1548–1553 (2005).
Goldman, A.L., van der Goes van Naters, W., Lessing, D., Warr, C.G. & Carlson, J.R. Coexpression of two functional odor receptors in one neuron. Neuron 45, 661–666 (2005).
Yao, C.A., Ignell, R. & Carlson, J.R. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J. Neurosci. 25, 8359–8367 (2005).
Hallem, E.A. & Carlson, J.R. Coding of odors by a receptor repertoire. Cell 125, 143–160 (2006).
Ng, M. et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 36, 463–474 (2002).
Wang, J.W., Wong, A.M., Flores, J., Vosshall, L.B. & Axel, R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112, 271–282 (2003).
Wilson, R.I., Turner, G.C. & Laurent, G. Transformation of olfactory representations in the Drosophila antennal lobe. Science 303, 366–370 (2004).
Shang, Y., Claridge-Chang, A., Sjulson, L., Pypaert, M. & Miesenbock, G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell 128, 601–612 (2007).
Derby, C.D. & Ache, B.W. Quality coding of a complex odorant in an invertebrate. J. Neurophysiol. 51, 906–924 (1984).
Anton, S. & Hansson, B.S. Central processing of sex pheromone, host odour, and oviposition deterrent information by interneurons in the antennal lobe of female Spodoptera littoralis (Lepidoptera: Noctuidae). J. Comp. Neurol. 350, 199–214 (1994).
Boeckh, J. & Boeckh, V. Threshold and odor specificity of pheromone-sensitive neurons in the deutocerebrum of Antheraea pernyi and A. polyphemus (Saturnidae). J. Comp. Physiol. [A] 132, 235–242 (1979).
Christensen, T.A., Mustaparta, H. & Hildebrand, J.G. Chemical communication in heliothine moths. II. Central processing of intra- and interspecific olfactory messages in the male corn earworm moth Heliocoverpa zea. J. Comp. Physiol. [A] 169, 259–274 (1991).
Vickers, N.J., Christensen, T.A. & Hildebrand, J.G. Combinatorial odor discrimination in the brain: attractive and antagonist odor blends are represented in distinct combinations of uniquely identifiable glomeruli. J. Comp. Neurol. 400, 35–56 (1998).
Guerenstein, P.G., Christensen, T.A. & Hildebrand, J.G. Sensory processing of ambient CO2 information in the brain of the moth Manduca sexta. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 190, 707–25 (2004).
Lin, D.Y., Zhang, S-Z., Block, E. & Katz, L.C. Encoding social signals in the mouse main olfactory bulb. Nature 434, 470–477 (2005).
Christensen, T.A. & Hildebrand, J.G. Pheromonal and host-odor processing in the insect antennal lobe: how different? Curr. Opin. Neurobiol. 12, 393–399 (2002).
Baxi, K.N., Dorries, K.M. & Eisthen, H.L. Is the vomeronasal system really specialized for detecting pheromones? Trends Neurosci. 29, 1–7 (2006).
Xu, P., Atkinson, R., Jones, D.N. & Smith, D.P. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron 45, 193–200 (2005).
Ha, T.S. & Smith, D.P. A pheromone receptor mediates 11-cis-vaccenyl acetate–induced responses in Drosophila. J. Neurosci. 26, 8727–8733 (2006).
Light, D.M., Jang, E.B., Binder, R.G., Flath, R.A. & Kint, S. Minor and intermediate components enhance attraction of female Mediterranean fruit flies to natural male odor pheromone and its synthetic major components. J. Chem. Ecol. 25, 2757–2777 (1999).
Vinje, W.E. & Gallant, J.L. Sparse coding and decorrelation in primary visual cortex during natural vision. Science 287, 1273–1276 (2000).
Bartelt, R.J., Schaner, A.M. & Jackson, L.L. cis-Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J. Chem. Ecol. 11, 1747–1756 (1985).
Jefferis, G.S. et al. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development 131, 117–130 (2004).
Marin, E.C., Jefferis, G.S., Komiyama, T., Zhu, H. & Luo, L. Representation of the glomerular olfactory map in the Drosophila brain. Cell 109, 243–255 (2002).
Marin, E.C., Watts, R.J., Tanaka, N.K., Ito, K. & Luo, L. Developmentally programmed remodeling of the Drosophila olfactory circuit. Development 132, 725–737 (2005).
Stockinger, P., Kvitsiani, D., Rotkopf, S., Tirian, L. & Dickson, B.J. Neural circuitry that governs Drosophila male courtship behavior. Cell 121, 795–807 (2005).
Manoli, D.S. et al. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 436, 395–400 (2005).
Dulac, C. & Wagner, S. Genetic analysis of brain circuits underlying pheromone signaling. Annu. Rev. Genet. 40, 449–467 (2006).
Shanbhag, S.R., Muller, B. & Steinbrecht, R.A. Atlas of olfactory organs of Drosophila melanogaster. 1. Types, external organization, innervation and distribution of olfactory sensilla. Int. J. Insect Morphol. Embryol. 28, 377–397 (1999).
Sankaranarayanan, S. & Ryan, T.A. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat. Cell Biol. 2, 197–204 (2000).
Pologruto, T.A., Yasuda, R. & Svoboda, K. Monitoring neural activity and [Ca2+] with genetically encoded Ca2+ indicators. J. Neurosci. 24, 9572–9579 (2004).
Wilson, R.I. & Laurent, G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J. Neurosci. 25, 9069–9079 (2005).
Fishilevich, E. et al. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr. Biol. 15, 2086–2096 (2005).
Stensmyr, M.C., Dekker, T. & Hansson, B.S. Evolution of the olfactory code in the Drosophila melanogaster subgroup. Proc. R. Soc. Lond. B 270, 2333–2340 (2003).
Acknowledgements
We are grateful to L. Vosshall, B. Dickson, L. Stevens and L. Luo for gifts of fly stocks, and to V. Bhandawat for help with single-sensillum recordings. C. Dulac and members of the Wilson lab provided feedback on earlier versions of the manuscript. Funding was provided by a Pew Scholarship in the Biomedical Sciences, a New Investigator Award from the Smith Family Foundation, an Armenise-Harvard Junior Faculty Grant, a Loreen Arbus Scholarship in Neuroscience and a grant from the US National Institutes of Health (RO1 1R01DC008174-01).
Author information
Authors and Affiliations
Contributions
This study was jointly designed by M.L.S. and R.I.W. The experiments and data analysis were performed by M.L.S., and M.L.S. and R.I.W. jointly wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
In Or67d-Gal4 flies, Gal4 is expressed ectopically in ab5A ORNs. (PDF 398 kb)
Supplementary Fig. 2
VA6 ORNs respond more strongly to geranyl acetate than to other highly related odors. (PDF 156 kb)
Rights and permissions
About this article
Cite this article
Schlief, M., Wilson, R. Olfactory processing and behavior downstream from highly selective receptor neurons. Nat Neurosci 10, 623–630 (2007). https://doi.org/10.1038/nn1881
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1881
This article is cited by
-
Sensorimotor transformation underlying odor-modulated locomotion in walking Drosophila
Nature Communications (2023)
-
The structural basis of odorant recognition in insect olfactory receptors
Nature (2021)
-
Morphology and physiology of olfactory neurons in the lateral protocerebrum of the silkmoth Bombyx mori
Scientific Reports (2019)
-
Chemical Cues that Guide Female Reproduction in Drosophila melanogaster
Journal of Chemical Ecology (2018)
-
Olfactory coding from the periphery to higher brain centers in the Drosophila brain
BMC Biology (2017)