Abstract
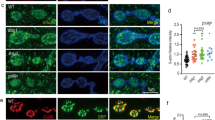
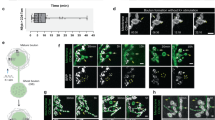
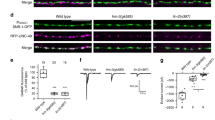
The morphological transition of growth cones to synaptic boutons characterizes synaptogenesis. Here we have isolated mutations in immaculate connections (imac; CG8566), a previously uncharacterized Drosophila gene encoding a member of the Kinesin-3 family. Whereas earlier studies in Drosophila implicated Kinesin-1 in transporting synaptic vesicle precursors, we find that Imac is essential for this transport. An unexpected feature of imac mutants is the failure of synaptic boutons to form. Motor neurons lacking imac properly target to muscles but remain within target fields as thin processes, a structure that is distinct from either growth cones or mature terminals. Few active zones form at these endings. We show that the arrest of synaptogenesis is not a secondary consequence of the absence of transmission. Our data thus indicate that Imac transports components required for synaptic maturation and provide insight into presynaptic maturation as a process that can be differentiated from axon outgrowth and targeting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Ziv, N.E. & Garner, C.C. Cellular and molecular mechanisms of presynaptic assembly. Nat. Rev. Neurosci. 5, 385–399 (2004).
Lee, H. & Van Vactor, D. Neurons take shape. Curr. Biol. 13, R152–R161 (2003).
Hannah, M.J., Schmidt, A.A. & Huttner, W.B. Synaptic vesicle biogenesis. Annu. Rev. Cell Dev. Biol. 15, 733–798 (1999).
Friedman, H.V., Bresler, T., Garner, C.C. & Ziv, N.E. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron 27, 57–69 (2000).
Roos, J. & Kelly, R.B. Preassembly and transport of nerve terminals: a new concept of axonal transport. Nat. Neurosci. 3, 415–417 (2000).
Ahmari, S.E., Buchanan, J. & Smith, S.J. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 3, 445–451 (2000).
Kraszewski, K. et al. Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J. Neurosci. 15, 4328–4342 (1995).
Shapira, M. et al. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron 38, 237–252 (2003).
Zhai, R.G. et al. Assembling the presynaptic active zone: a characterization of an active zone precursor vesicle. Neuron 29, 131–143 (2001).
Sanes, J.R. & Lichtman, J.W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 22, 389–442 (1999).
Featherstone, D.E. & Broadie, K. Surprises from Drosophila: genetic mechanisms of synaptic development and plasticity. Brain Res. Bull. 53, 501–511 (2000).
Yoshihara, M., Rheuben, M.B. & Kidokoro, Y. Transition from growth cone to functional motor nerve terminal in Drosophila embryos. J. Neurosci. 17, 8408–8426 (1997).
Rheuben, M.B., Yoshihara, M. & Kidokoro, Y. Ultrastructural correlates of neuromuscular junction development. Int. Rev. Neurobiol. 43, 69–92 (1999).
Stowers, R.S. & Schwarz, T.L. A Genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics 152, 1631–1639 (1999).
Dickman, D.K., Horne, J.A., Meinertzhagen, I.A. & Schwarz, T.L. A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell 123, 521–533 (2005).
Chang, T.N. & Keshishian, H. Laser ablation of Drosophila embryonic motoneurons causes ectopic innervation of target muscle fibers. J. Neurosci. 16, 5715–5726 (1996).
Berger, J. et al. Genetic mapping with SNP markers in Drosophila. Nat. Genet. 29, 475–481 (2001).
Loren, C.E. et al. A crucial role for the Anaplastic lymphoma kinase receptor tyrosine kinase in gut development in Drosophila melanogaster. EMBO Rep. 4, 781–786 (2003).
Vale, R.D. The molecular motor toolbox for intracellular transport. Cell 112, 467–480 (2003).
Broadie, K.S. & Bate, M. Development of the embryonic neuromuscular synapse of Drosophila melanogaster. J. Neurosci. 13, 144–166 (1993).
Hall, D.H. & Hedgecock, E.M. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65, 837–847 (1991).
Okada, Y., Yamazaki, H., Sekine-Aizawa, Y. & Hirokawa, N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell 81, 769–780 (1995).
Zhao, C. et al. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bβ. Cell 105, 587–597 (2001).
Hurd, D.D. & Saxton, W.M. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics 144, 1075–1085 (1996).
Goldstein, L.S. & Yang, Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu. Rev. Neurosci. 23, 39–71 (2000).
Jacob, T.C. & Kaplan, J.M. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J. Neurosci. 23, 2122–2130 (2003).
Rao, S., Lang, C., Levitan, E.S. & Deitcher, D.L. Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J. Neurobiol. 49, 159–172 (2001).
Murthy, M., Garza, D., Scheller, R.H. & Schwarz, T.L. Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron 37, 433–447 (2003).
Lin, D.M. & Goodman, C.S. Ectopic and increased expression of fasciclin II alters motoneuron growth cone guidance. Neuron 13, 507–523 (1994).
Burgess, R.W., Deitcher, D.L. & Schwarz, T.L. The synaptic protein syntaxin1 is required for cellularization of Drosophila embryos. J. Cell Biol. 138, 861–875 (1997).
Glater, E.E., Megeath, L.J., Stowers, R.S. & Schwarz, T.L. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 173, 545–557 (2006).
Pilling, A.D., Horiuchi, D., Lively, C.M. & Saxton, W.M. Kinesin-1 and dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell 17, 2057–2068 (2006).
Roos, J., Hummel, T., Ng, N., Klambt, C. & Davis, G.W. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron 26, 371–382 (2000).
Ma, D., Himes, B.T., Shea, T.B. & Fischer, I. Axonal transport of microtubule-associated protein 1B (MAP1B) in the sciatic nerve of adult rat: distinct transport rates of different isoforms. J. Neurosci. 20, 2112–2120 (2000).
Schwarz, T.L. Transmitter release at the neuromuscular junction. Int. Rev. Neurobiol. 75, 105–144 (2006).
Wagh, D.A. et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 49, 833–844 (2006).
Broadie, K. et al. Syntaxin and synaptohrevin function downstream of vesicle docking in Drosophila. Neuron 15, 663–673 (1995).
Featherstone, D.E., Rushton, E. & Broadie, K. Developmental regulation of glutamate receptor field size by nonvesicular glutamate release. Nat. Neurosci. 5, 141–146 (2002).
DiAntonio, A. Glutamate receptors at the Drosophila neuromuscular junction. Int. Rev. Neurobiol. 75, 165–179 (2006).
Dickson, B.J. Molecular mechanisms of axon guidance. Science 298, 1959–1964 (2002).
Marques, G. & Zhang, B. Retrograde signaling that regulates synaptic development and function at the Drosophila neuromuscular junction. Int. Rev. Neurobiol. 75, 267–285 (2006).
Shen, K., Fetter, R.D. & Bargmann, C.I. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell 116, 869–881 (2004).
Ackley, B.D. & Jin, Y. Genetic analysis of synaptic target recognition and assembly. Trends Neurosci. 27, 540–547 (2004).
Dickman, D.K., Lu, Z., Meinertzhagen, I.A. & Schwarz, T.L. Altered synaptic development and active zone spacing in endocytosis mutants. Curr. Biol. 16, 591–598 (2006).
Klopfenstein, D.R., Tomishige, M., Stuurman, N. & Vale, R.D. Role of phosphatidylinositol(4,5)bisphosphate organization in membrane transport by the Unc104 kinesin motor. Cell 109, 347–358 (2002).
Sato-Yoshitake, R., Yorifuji, H., Inagaki, M. & Hirokawa, N. The phosphorylation of kinesin regulates its binding to synaptic vesicles. J. Biol. Chem. 267, 23930–23936 (1992).
Takamori, S. et al. Molecular anatomy of a trafficking organelle. Cell 127, 831–846 (2006).
Miller, K.E. et al. Direct observation demonstrates that Liprin-α is required for trafficking of synaptic vesicles. Curr. Biol. 15, 684–689 (2005).
Saitoe, M., Schwarz, T.L., Umbach, J.A., Gundersen, C.B. & Kidokoro, Y. Absence of junctional glutamate receptor clusters in Drosophila mutants lacking spontaneous transmitter release. Science 293, 514–517 (2001).
Prokop, A. & Technau, G.M. in Cellular Interactions in Development: A Practical Approach (ed. Hartley, D.) 33–57 (Oxford Univ. Press, London and New York, 1993).
Acknowledgements
We thank W. Saxton, D. Van Vactor and J. Kaplan for comments on the manuscript; A. DiAntonio, R. Palmer, N. Reist, M. Higashi, and the Bloomington Stock Center for reagents and fly strains; A.Y.N. Goldstein and J. Salogiannis for experimental assistance; members of the Schwarz laboratory for discussions; HCNR and DDRC imaging cores for imaging and analysis assistance; A. Prokop and the HMS electron microscopy facility for electron microscopy help. This work was supported by a US National Research Service Award predoctoral fellowship (E.P.-C.), a Howard Hughes Medical Institute predoctoral fellowship (D.K.D.), and a grant from the US National Institutes of Health (RO1-MH075058 to T.L.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Table 1, Methods (PDF 9697 kb)
Rights and permissions
About this article
Cite this article
Pack-Chung, E., Kurshan, P., Dickman, D. et al. A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat Neurosci 10, 980–989 (2007). https://doi.org/10.1038/nn1936
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1936
This article is cited by
-
Liprin-α proteins are master regulators of human presynapse assembly
Nature Neuroscience (2024)
-
Synapse development and maturation at the drosophila neuromuscular junction
Neural Development (2020)
-
A method for estimating relative changes in the synaptic density in Drosophila central nervous system
BMC Neuroscience (2018)
-
The KIF1A homolog Unc-104 is important for spontaneous release, postsynaptic density maturation and perisynaptic scaffold organization
Scientific Reports (2017)
-
The intricate relationship between microtubules and their associated motor proteins during axon growth and maintenance
Neural Development (2013)