Abstract
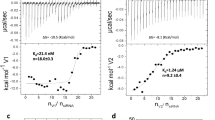
RNA nanoparticles have applications in the treatment of cancers and viral infection; however, the instability of RNA nanoparticles has hindered their development for therapeutic applications. The lack of covalent linkage or crosslinking in nanoparticles causes dissociation in vivo. Here we show that the packaging RNA of bacteriophage phi29 DNA packaging motor can be assembled from 3–6 pieces of RNA oligomers without the use of metal salts. Each RNA oligomer contains a functional module that can be a receptor-binding ligand, aptamer, short interfering RNA or ribozyme. When mixed together, they self-assemble into thermodynamically stable tri-star nanoparticles with a three-way junction core. These nanoparticles are resistant to 8 M urea denaturation, are stable in serum and remain intact at extremely low concentrations. The modules remain functional in vitro and in vivo, suggesting that the three-way junction core can be used as a platform for building a variety of multifunctional nanoparticles. We studied 25 different three-way junction motifs in biological RNA and found only one other motif that shares characteristics similar to the three-way junction of phi29 pRNA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Seeman, N. C. Nanomaterials based on DNA. Annu. Rev. Biochem. 79, 65–87 (2010).
Guo, P. The emerging field of RNA nanotechnology. Nature Nanotech. 5, 833–842 (2010).
Guo, P. et al. Inter-RNA interaction of phage phi29 pRNA to form a hexameric complex for viral DNA transportation. Mol. Cell. 2, 149–155 (1998).
Shukla, G. C. et al. A boost for the emerging field of RNA nanotechnology. ACS Nano 5, 3405–3418 (2011).
Cruz, J. A. & Westhof, E. The dynamic landscapes of RNA architecture. Cell 136, 604–609 (2009).
Jaeger, L., Verzemnieks, E. J. & Geary, C. The UA_handle: a versatile submotif in stable RNA architectures. Nucleic Acids Res. 37, 215–230 (2009).
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998).
Khaled, A., Guo, S., Li, F. & Guo, P. Controllable self-assembly of nanoparticles for specific delivery of multiple therapeutic molecules to cancer cells using RNA nanotechnology. Nano Lett. 5, 1797–1808 (2005).
Guo, S., Tschammer, N., Mohammed, S. & Guo, P. Specific delivery of therapeutic RNAs to cancer cells via the dimerization mechanism of phi29 motor pRNA. Hum. Gene Ther. 16, 1097–1109 (2005).
Guo, S., Huang, F. & Guo, P. Construction of folate-conjugated pRNA of bacteriophage phi29 DNA packaging motor for delivery of chimeric siRNA to nasopharyngeal carcinoma cells. Gene Ther. 13, 814–820 (2006).
Hoeprich, S. et al. Bacterial virus phi29 pRNA as a hammerhead ribozyme escort to destroy hepatitis B virus. Gene Ther. 10, 1258–1267 (2003).
Sarver, N. A. et al. Ribozymes as potential anti-HIV-1 therapeutic agents. Science 247, 1222–1225 (1990).
Liu, H. et al. Phi29 pRNA vector for efficient escort of hammerhead ribozyme targeting survivin in multiple cancer cells. Cancer Biol. Ther. 6, 697–704 (2007).
Winkler, W. C. et al. Control of gene expression by a natural metabolite-responsive ribozyme. Nature 428, 281–286 (2004).
Mulhbacher, J., St-Pierre, P. & Lafontaine, D. A. Therapeutic applications of ribozymes and riboswitches. Curr. Opin. Pharmacol. 10, 551–556 (2010).
Chen, Y. et al. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol. Ther. 18, 1650–1656 (2010).
Pegtel, D. M. et al. Functional delivery of viral miRNAs via exosomes. Proc. Natl Acad. Sci. USA 107, 6328–6333 (2010).
Ye, X., Liu, Z., Hemida, M. & Yang, D. Mutation tolerance and targeted delivery of anti-coxsackievirus artificial MicroRNAs using folate conjugated bacteriophage Phi29 pRNA. PLoS ONE 6, e21215 (2011).
Chen, C. & Guo, P. Magnesium-induced conformational change of packaging RNA for procapsid recognition and binding during phage phi29 DNA encapsidation. J. Virol. 71, 495–500 (1997).
Chen, C., Sheng, S., Shao, Z. & Guo, P. A dimer as a building block in assembling RNA: a hexamer that gears bacterial virus phi29 DNA-translocating machinery. J. Biol. Chem. 275, 17510–17516 (2000).
Guo, P., Erickson, S. & Anderson, D. A small viral RNA is required for in vitro packaging of bacteriophage phi29 DNA. Science 236, 690–694 (1987).
Reid, R. J. D., Bodley, J. W. & Anderson, D. Characterization of the prohead-pRNA interaction of bacteriophage phi29. J. Biol. Chem. 269, 5157–5162 (1994).
Zhang, C. L., Lee, C.-S. & Guo, P. The proximate 5′ and 3′ ends of the 120-base viral RNA (pRNA) are crucial for the packaging of bacteriophage φ29 DNA. Virology 201, 77–85 (1994).
Shu, D., Zhang, H., Jin, J. & Guo, P. Counting of six pRNAs of phi29 DNA-packaging motor with customized single molecule dual-view system. EMBO J. 26, 527–537 (2007).
Xiao, F., Moll, D., Guo, S. & Guo, P. Binding of pRNA to the N-terminal 14 amino acids of connector protein of bacterial phage phi29. Nucleic Acids Res. 33, 2640–2649 (2005).
Shu, D. et al. Bottom-up assembly of RNA arrays and superstructures as potential parts in nanotechnology. Nano Lett. 4, 1717–1723 (2004).
Zhang, C. L., Trottier, M. & Guo, P. X. Circularly permuted viral pRNA active and specific in the packaging of bacteriophage Phi29 DNA. Virology 207, 442–451 (1995).
Carlson, R. D., Olins, A. L. & Olins, D. E. Urea denaturation of chromatin periodic structure. Biochemistry 14, 3122–3125 (1975).
Pagratis, N. C. Rapid preparation of single stranded DNA from PCR products by streptavidin induced electrophoretic mobility shift. Nucleic Acids Res. 24, 3645–3646 (1996).
Baugh, C., Grate, D. & Wilson, C. 2.8 Å crystal structure of the malachite green aptamer. J. Mol. Biol. 301, 117–128 (2000).
Lu, Y. & Low, P. S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Deliv. Rev. 54, 675–693 (2002).
Ambrosini, G., Adida, C. & Altieri, D. C. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nature Med. 3, 917–921 (1997).
Soutschek, J. et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432, 173–178 (2004).
Behlke, M. A. Progress towards in vivo use of siRNAs. Mol. Ther. 13, 644–670 (2006).
Chen, C., Zhang, C. & Guo, P. Sequence requirement for hand-in-hand interaction in formation of pRNA dimers and hexamers to gear phi29 DNA translocation motor. RNA 5, 805–818 (1999).
de la, P. M., Dufour, D. & Gallego, J. Three-way RNA junctions with remote tertiary contacts: a recurrent and highly versatile fold. RNA. 15, 1949–1964 (2009).
Lilley, D. M. Structures of helical junctions in nucleic acids. Q. Rev. Biophys. 33, 109–159 (2000).
Afonin, K. A. et al. In vitro assembly of cubic RNA-based scaffolds designed in silico. Nature Nanotech. 5, 676–682 (2010).
Lescoute, A. & Westhof, E. Topology of three-way junctions in folded RNAs. RNA 12, 83–93 (2006).
Leontis, N. B. & Westhof, E. Analysis of RNA motifs. Curr. Opin. Struct. Biol. 13, 300–308 (2003).
Honda, M., Beard, M. R., Ping, L. H. & Lemon, S. M. A phylogenetically conserved stem-loop structure at the 5′ border of the internal ribosome entry site of hepatitis C virus is required for cap-independent viral translation. J. Virol. 73, 1165–1174 (1999).
Wakeman, C. A., Ramesh, A. & Winkler, W. C. Multiple metal-binding cores are required for metalloregulation by M-box riboswitch RNAs. J. Mol. Biol. 392, 723–735 (2009).
Kulshina, N., Edwards, T. E. & Ferre-D'Amare, A. R. Thermodynamic analysis of ligand binding and ligand binding-induced tertiary structure formation by the thiamine pyrophosphate riboswitch. RNA 16, 186–196 (2010).
Diamond, J. M., Turner, D. H. & Mathews, D. H. Thermodynamics of three-way multibranch loops in RNA. Biochemistry 40, 6971–6981 (2001).
Klostermeier, D. & Millar, D. P. Helical junctions as determinants for RNA folding: origin of tertiary structure stability of the hairpin ribozyme. Biochemistry 39, 12970–12978 (2000).
Rettberg, C. C. et al. A three-way junction and constituent stem-loops as the stimulator for programmed -1 frameshifting in bacterial insertion sequence IS911. J. Mol. Biol. 286, 1365–1378 (1999).
Liu, B., Diamond, J. M., Mathews, D. H. & Turner, D. H. Fluorescence competition and optical melting measurements of RNA three-way multibranch loops provide a revised model for thermodynamic parameters. Biochemistry 50, 640–653 (2011).
Mathews, D. H. & Turner, D. H. Experimentally derived nearest-neighbor parameters for the stability of RNA three- and four-way multibranch loops. Biochemistry 41, 869–880 (2002).
Abdelmawla, S. et al. Pharmacological characterization of chemically synthesized monomeric pRNA nanoparticles for systemic delivery. Mol. Ther. 19, 1312–1322 (2011).
Lyubchenko, Y. L. & Shlyakhtenko, L. S. AFM for analysis of structure and dynamics of DNA and protein-DNA complexes. Methods 47, 206–213 (2009).
Shu, Y. et al. Assembly of therapeutic pRNA-siRNA nanoparticles using bipartite approach. Mol. Ther. 19, 1304–1311 (2011).
Acknowledgements
This research was mainly supported by the National Institutes of Health (NIH; grants EB003730, GM059944 and CA151648 to P.G.). P.G. is also a co-founder of Kylin Therapeutics Inc. The authors thank L. Shlyakhtenko and Y. Lyubchenko for AFM images via the Nanoimaging Core Facility supported by the NIH SIG Program and the UNMC Program of ENRI, as well as N. Abdeltawab and Z. Zhu from M. Kotb's laboratory at the University of Cincinnati for help with qRT–PCR assays.
Author information
Authors and Affiliations
Contributions
P.G. conceived, designed and led the project. D.S., Y.S. and F.H. designed and conducted the in vitro experiments. S.A. performed animal imaging experiments. P.G., D.S., Y.S. and F.H. analysed the data and co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1901 kb)
Rights and permissions
About this article
Cite this article
Shu, D., Shu, Y., Haque, F. et al. Thermodynamically stable RNA three-way junction for constructing multifunctional nanoparticles for delivery of therapeutics. Nature Nanotech 6, 658–667 (2011). https://doi.org/10.1038/nnano.2011.105
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2011.105
This article is cited by
-
Diverse applications and development of aptamer detection technology
Analytical Sciences (2023)
-
Multi-arm RNA junctions encoding molecular logic unconstrained by input sequence for versatile cell-free diagnostics
Nature Biomedical Engineering (2022)