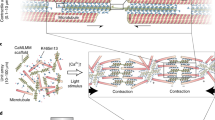
Abstract
Biomolecular motors such as myosin, kinesin and dynein are protein machines that can drive directional movement along cytoskeletal tracks1,2 and have the potential to be used as molecule-sized actuators3,4. Although control of the velocity and directionality of biomolecular motors has been achieved5,6,7,8, the design and construction of novel biomolecular motors remains a challenge. Here we show that naturally occurring protein building blocks from different cytoskeletal systems can be combined to create a new series of biomolecular motors. We show that the hybrid motors—combinations of a motor core derived from the microtubule-based dynein motor and non-motor actin-binding proteins—robustly drive the sliding movement of an actin filament. Furthermore, the direction of actin movement can be reversed by simply changing the geometric arrangement of these building blocks. Our synthetic strategy provides an approach to fabricating biomolecular machines that work along artificial tracks at nanoscale dimensions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vale, R. D. The molecular motor toolbox for intracellular transport. Cell 112, 467–480 (2003).
Howard, J. Mechanics of Motor Proteins and the Cytoskeleton 1st edn (Sinauer Associates, 2001).
van den Heuvel, M. G. & Dekker, C. Motor proteins at work for nanotechnology. Science 317, 333–336 (2007).
Diez, S., Helenius, J. H. & Howard, J. in Protein Science Encyclopedia 185–199 (Wiley, 2008).
Tsiavaliaris, G., Fujita-Becker, S. & Manstein, D. J. Molecular engineering of a backwards-moving myosin motor. Nature 427, 558–561 (2004).
Liu, H. et al. Control of a biomolecular motor-powered nanodevice with an engineered chemical switch. Nat. Mater. 1, 173–177 (2002).
Nomura, A., Uyeda, T. Q., Yumoto, N. & Tatsu, Y. Photo-control of kinesin-microtubule motility using caged peptides derived from the kinesin C-terminus domain. Chem. Commun. 3588–3590 (2006).
Chen, L., Nakamura, M., Schindler, T. D., Parker, D. & Bryant, Z. Engineering controllable bidirectional molecular motors based on myosin. Nat. Nanotech. 7, 252–256 (2012).
Hackney, D. D. The kinetic cycles of myosin, kinesin, and dynein. Annu. Rev. Physiol. 58, 731–750 (1996).
Howard, J. Protein power strokes. Curr. Biol. 16, R517–R519 (2006).
Kon, T., Mogami, T., Ohkura, R., Nishiura, M. & Sutoh, K. ATP hydrolysis cycle-dependent tail motions in cytoplasmic dynein. Nat. Struct. Mol. Biol. 12, 513–519 (2005).
Roberts, A. J., Kon, T., Knight, P. J., Sutoh, K. & Burgess, S. A. Functions and mechanics of dynein motor proteins. Nat. Rev. Mol. Cell Biol. 14, 713–726 (2013).
Vallee, R. B., Williams, J. C., Varma, D. & Barnhart, L. E. Dynein: an ancient motor protein involved in multiple modes of transport. J. Neurobiol. 58, 189–200 (2004).
Gibbons, I. R. Cilia and flagella of eukaryotes. J. Cell Biol. 91, 107s–124s (1981).
Burgess, S. A., Walker, M. L., Sakakibara, H., Knight, P. J. & Oiwa, K. Dynein structure and power stroke. Nature 421, 715–718 (2003).
Roberts, A. J. et al. AAA+ Ring and linker swing mechanism in the dynein motor. Cell 136, 485–495 (2009).
Kon, T. et al. The 2.8 Å crystal structure of the dynein motor domain. Nature 484, 345–350 (2012).
Schmidt, H., Zalyte, R., Urnavicius, L. & Carter, A. P. Structure of human cytoplasmic dynein-2 primed for its power stroke. Nature 518, 435–438 (2015).
Carter, A. P. et al. Structure and functional role of dynein's microtubule-binding domain. Science 322, 1691–1695 (2008).
Redwine, W. B. et al. Structural basis for microtubule binding and release by dynein. Science 337, 1532–1536 (2012).
Shima, T., Kon, T., Imamula, K., Ohkura, R. & Sutoh, K. Two modes of microtubule sliding driven by cytoplasmic dynein. Proc. Natl Acad. Sci. USA 103, 17736–17740 (2006).
Gibbons, I. R. et al. The affinity of the dynein microtubule-binding domain is modulated by the conformation of its coiled-coil stalk. J. Biol. Chem. 280, 23960–23965 (2005).
Kon, T. et al. Helix sliding in the stalk coiled coil of dynein couples ATPase and microtubule binding. Nat. Struct. Mol. Biol. 16, 325–333 (2009).
Nishikawa, Y., Inatomi, M., Iwasaki, H. & Kurisu, G. Structural change in the dynein stalk region associated with two different affinities for the microtubule. J. Mol. Biol. 428, 1886–1896 (2015).
Burgess, S. A. & Knight, P. J. Is the dynein motor a winch? Curr. Opin. Struct. Biol. 14, 138–146 (2004).
Kim, L. Y. et al. The structural basis of actin organization by vinculin and metavinculin. J. Mol. Biol. 428, 10–25 (2015).
Vale, R. D. & Oosawa, F. Protein motors and Maxwell's demons: does mechanochemical transduction involve a thermal ratchet? Adv. Biophys. 26, 97–134 (1990).
Cleary, F. B. et al. Tension on the linker gates the ATP-dependent release of dynein from microtubules. Nat. Commun. 5, 4587 (2014).
Bissell, R. A., Córdova, E., Kaifer, A. E. & Stoddart, J. F. A chemically and electrochemically switchable molecular shuttle. Nature 369, 133–137 (1994).
Bath, J. & Turberfield, A. J. DNA nanomachines. Nat. Nanotech. 2, 275–284 (2007).
Reck-Peterson, S. L. et al. Single-molecule analysis of dynein processivity and stepping behavior. Cell 126, 335–348 (2006).
Jacob, F. Evolution and tinkering. Science 196, 1161–1166 (1977).
Uchimura, S. et al. A flipped ion pair at the dynein-microtubule interface is critical for dynein motility and ATPase activation. J. Cell Biol. 208, 211–222 (2015).
Baird, G. S., Zacharias, D. A. & Tsien, R. Y. Circular permutation and receptor insertion within Green fluorescent proteins. Proc. Natl Acad. Sci. USA 96, 11241–11246 (1999).
Martin, A., Baker, T. A. & Sauer, R. T. Rebuilt AAA + motors reveal operating principles for ATP-fuelled machines. Nature 437, 1115–1120 (2005).
Torisawa, T. et al. Autoinhibition and cooperative activation mechanisms of cytoplasmic dynein. Nat. Cell Biol. 16, 1118–1124 (2014).
Spudich, J. A. & Watt, S. The regulation of rabbit skeletal muscle contraction. I: biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 246, 4866–4871 (1971).
Herm-Gotz, A. et al. Toxoplasma gondii myosin A and its light chain: a fast, single-headed, plus-end-directed motor. EMBO J. 21, 2149–2158 (2002).
Perry, S. V. Myosin adenosine triphosphatase. Meth. Enzymol. 2, 582–588 (1955).
Hyman, A. A. Preparation of marked microtubules for the assay of the polarity of microtubule-based motors by fluorescence. J. Cell Sci. Suppl. 14, 125–127 (1991).
Suzuki, N., Miyata, H., Ishiwata, S. & Kinosita, K. Jr. Preparation of bead-tailed actin filaments: estimation of the torque produced by the sliding force in an in vitro motility assay. Biophys. J. 70, 401–408 (1996).
Furuta, K. et al. Measuring collective transport by defined numbers of processive and nonprocessive kinesin motors. Proc. Natl Acad. Sci. USA 110, 501–506 (2013).
Edelstein, A., Amodaj, N., Hoover, K., Vale, R. & Stuurman, N. Computer control of microscopes using µManager. Curr. Protoc. Mol. Biol. 92, 14.20.1–14.20.17 (2010).
Acknowledgements
We thank Y. Watari and K. Yamamoto (NICT, Kobe, Japan) for technical assistance, C. Mori, K. Okamasa, Y. Nagahama and N. Fukuta (NICT, Kobe, Japan) for DNA sequencing and K. Miura (EMBL, Germany) for the Temporal-Color Code ImageJ plugin. This work was supported by a Grant-in-Aid for Young Scientists B from the Ministry of Education, Culture, Sports, Science and Technology, Japan (grant number 22770164 to K.F.) a Grant-in-Aid for Scientific Research C from the Japan Society for the Promotion of Science (grant number 26440089 to K.O.) and the Takeda Science Foundation (to K.O.).
Author information
Authors and Affiliations
Contributions
A.F. and K.F. conceived and designed the project. A.F., M.A., M.Y. and K.F. conducted the experiments. All authors contributed to writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2317 kb)
Supplementary information
Supplementary Movie 1 (MP4 7420 kb)
Supplementary information
Supplementary Movie 2 (MP4 146 kb)
Supplementary information
Supplementary Movie 3 (MP4 316 kb)
Supplementary information
Supplementary Movie 4 (MP4 436 kb)
Supplementary information
Supplementary Movie 5 (MP4 213 kb)
Supplementary information
Supplementary Movie 6 (MP4 651 kb)
Supplementary information
Supplementary Movie 7 (MP4 1431 kb)
Supplementary information
Supplementary Movie 8 (MP4 3803 kb)
Supplementary information
Supplementary Movie 9 (MP4 826 kb)
Supplementary information
Supplementary Movie 10 (MP4 3418 kb)
Supplementary information
Supplementary Movie 11 (MP4 3258 kb)
Rights and permissions
About this article
Cite this article
Furuta, A., Amino, M., Yoshio, M. et al. Creating biomolecular motors based on dynein and actin-binding proteins. Nature Nanotech 12, 233–237 (2017). https://doi.org/10.1038/nnano.2016.238
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2016.238
This article is cited by
-
Optical control of fast and processive engineered myosins in vitro and in living cells
Nature Chemical Biology (2021)
-
Synthetic biology approaches to dissecting linear motor protein function: towards the design and synthesis of artificial autonomous protein walkers
Biophysical Reviews (2020)
-
Kinesin-6 Klp9 plays motor-dependent and -independent roles in collaboration with Kinesin-5 Cut7 and the microtubule crosslinker Ase1 in fission yeast
Scientific Reports (2019)
-
Directionality of dynein is controlled by the angle and length of its stalk
Nature (2019)
-
AMPK-dependent phosphorylation of cingulin reversibly regulates its binding to actin filaments and microtubules
Scientific Reports (2018)